answer 8.2 - use the ligand binding polynomial to fit the model to the esperimental data
The equation for the fraction of X-liganded sites, for every concentration of X and Y:
([PX]+[PYX])/[P]tot = [X]Ky(xKy + [Y]) / [KxxKy (Ky + [Y]) + [X]Ky(xKy + [Y])]
yields a nice fit of the experimental data with the following parameters: Kx = 1 mM; Ky = 0.3 mM; xKy = 1.5 mM. We easily calculate that yKx = 5 mM.
The plot of X50 versus [Y] yields a hyperbola with X50=1 mM at [Y]=0, and upper asymptote of X50=5 mM at [Y]>>Ky+xKy. The abscissa of the midpoint is intermediate between Ky and xKy. Notice that this scheme allows for xKy > Ky (negative heterotropic linkage) and xKy < Ky (positive heterotropic linkage).
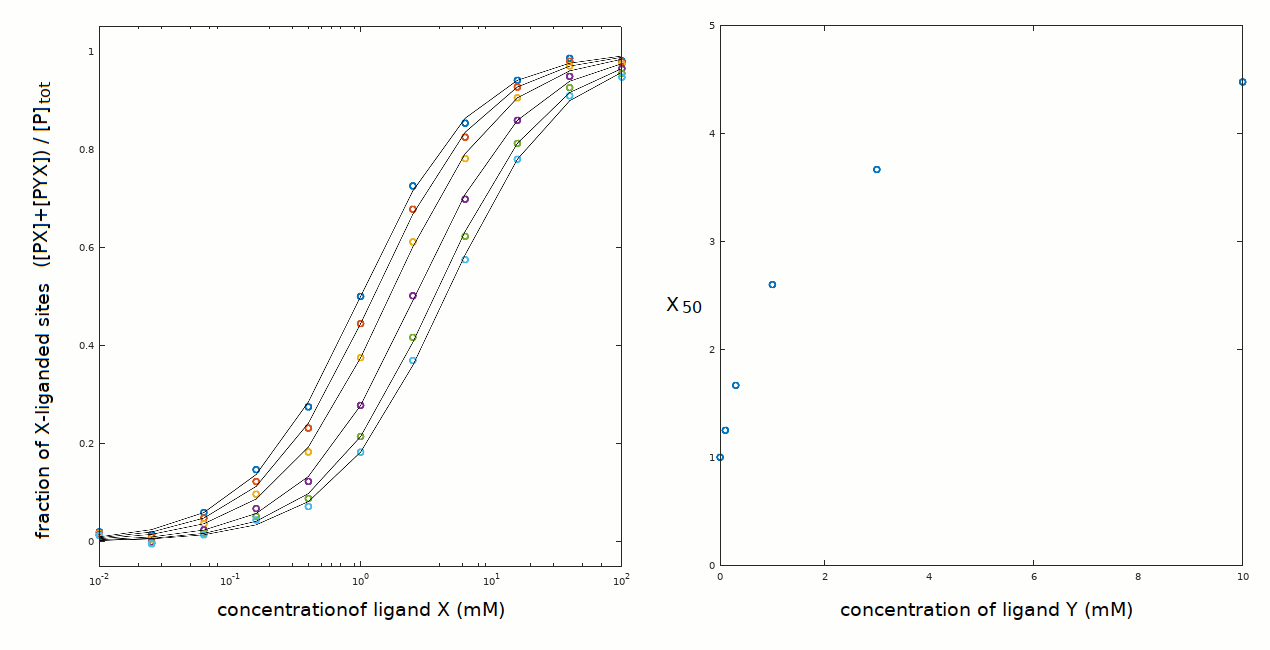
The midpoint of the hyperbolic function X50 versus [Y] has coordinates X50 = Kx 0.5(1 + xKy/Ky) and [Y] = xKy. For example, in the present case the coordinates of the midpoint are X50 = 1x0.5x(1 + 1.5/0.3) = 3 mM and [Y]=1.5 mM. We can reconstruct the ligand binding polynomial for the above constants and concentrations of X and Y and obtain:
[P]tot = [P] (0.45 + 1,35 + 2.25 + 1.35) / 0.45
fraction of X bound protein: ([PX] + [PYX]) / [P]tot = (1.35 + 1.35) / (0.45 + 1.35 + 2.25 + 1.35 ) = 0.5
fraction of Y bound protein: (2.25 + 1.35) / (0.45 + 1.35 + 2.25 + 1.35 ) = 0.67
We remark that the degree of saturation of the protein with ligand Y (kept at constant concentration) varies during the titration with ligand X, and precisely it increases with increasing X in the case of positive heterotropic linkage and decreases in the case of negative heterotropic linkage. However, contrary to the case of identical linkage, the protein maintains some bound Y throughout.
One may take advantage of heterotropic linkage to solve some special problems that may be related to the measurement of ligand binding constants:
1) heterotropic linkage allows one to measure the affinity of ligand Y by recording its effect on X50. Thus one may study ligands whose binding yields no signal (e.g. proton binding to hemoglobin via their effect on oxygen affinity).
2) Negative heterotropic linkage may be resorted to in the case of high and very high affinity ligands (ligand Y decreases the affinity of the protein for ligand X).
Back one step or back to main index.