|
Andrea Bellelli Dipartimento di Scienze Biochimiche "A. Rossi Fanelli", Sapienza Universita' di Roma Some important facts about schistosomiasis: - Schistosomes are important human parasites, responsible for a disease that affects over 200 million people mainly in tropical countries. - The parasite is a flatworm whose life cycle requires a freshwater mollusc as the intermediate host. Thus, the disease is more common in areas where freshwater is available, i.e. areas that are important for farming and are densely inhabited. - Only one drug, praziquantel, is used to treat the infection. Although the drug is effective, less sensitive strains are emerging. - The disease does not confer significative immunity and reinfections are frequent. 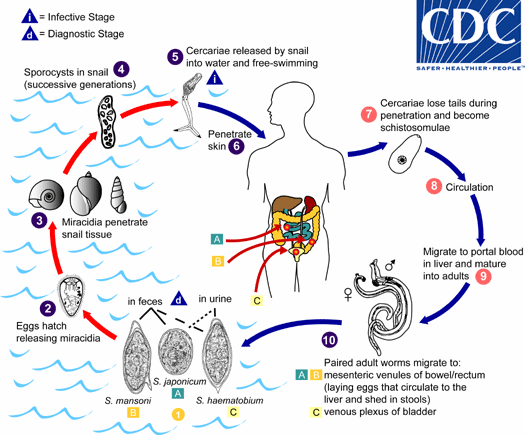
Figura 1: ciclo vitale dello Schistosoma 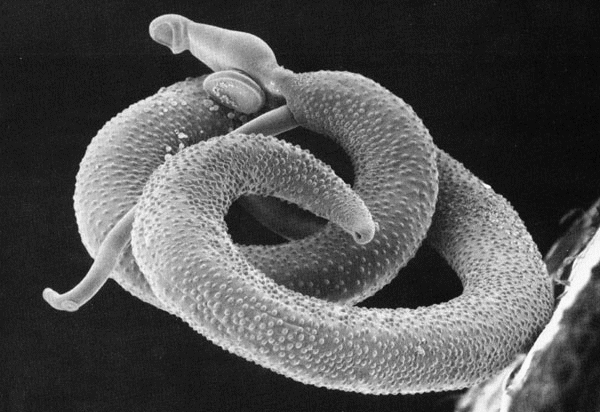
Figura 2: una coppia di Schistosomi (la femmina e' ospitata nel canale ginecoforo del maschio) vista col microscopio elettronico a scansione 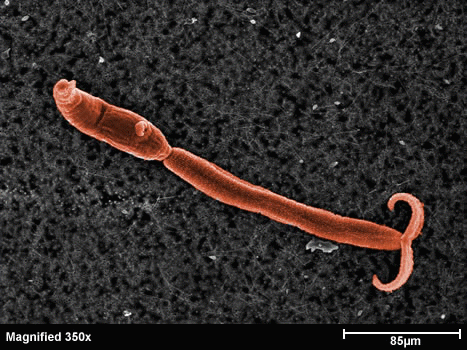
Figura 3: la larva cercaria - Glutathione S-transferase (GST) - Cyclophilin (Cyp) - Fatty Acid Binding Protein (FABP) - Thioredoxin Glutathione Reductase (TGR) - Thioredoxin (Trx) - Glutathione peroxidase (Gpx) - Thioredoxin peroxidase (peroxiredoxin, Prx) - In schistosomes and other parasites only one enzyme (TGR) reduces both glutathione and thioredoxin. This is at variance with the great majority of animal cells that use different enzymes (glutathione reductase and thioredoxin reductase) - This peculiarity suggests that TGR may be a specific target for antiparasitic therapy We solved the structure of TGR from S. mansoni using X-ray crystallography. Salient structural features are: - the protein is a homodimer; - each monomer has two structural domains: one glutaredoxin-like, and one thioredoxin reductase-like; - each monomer contains one FAD; - each monomer has at least one redox-active Cys couple (in front of the FAD) and a redox-active Sec-Cys couple in the C-terminal arm. Other active Cys residues are present on both the Grx domain and the TrxR domain. 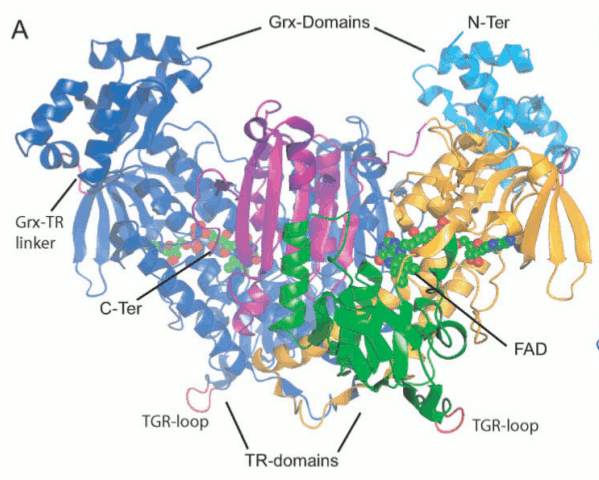
Figura 4: Structure of Thioredoxin Glutathione Reductase (TGR) from Schistosoma mansoni 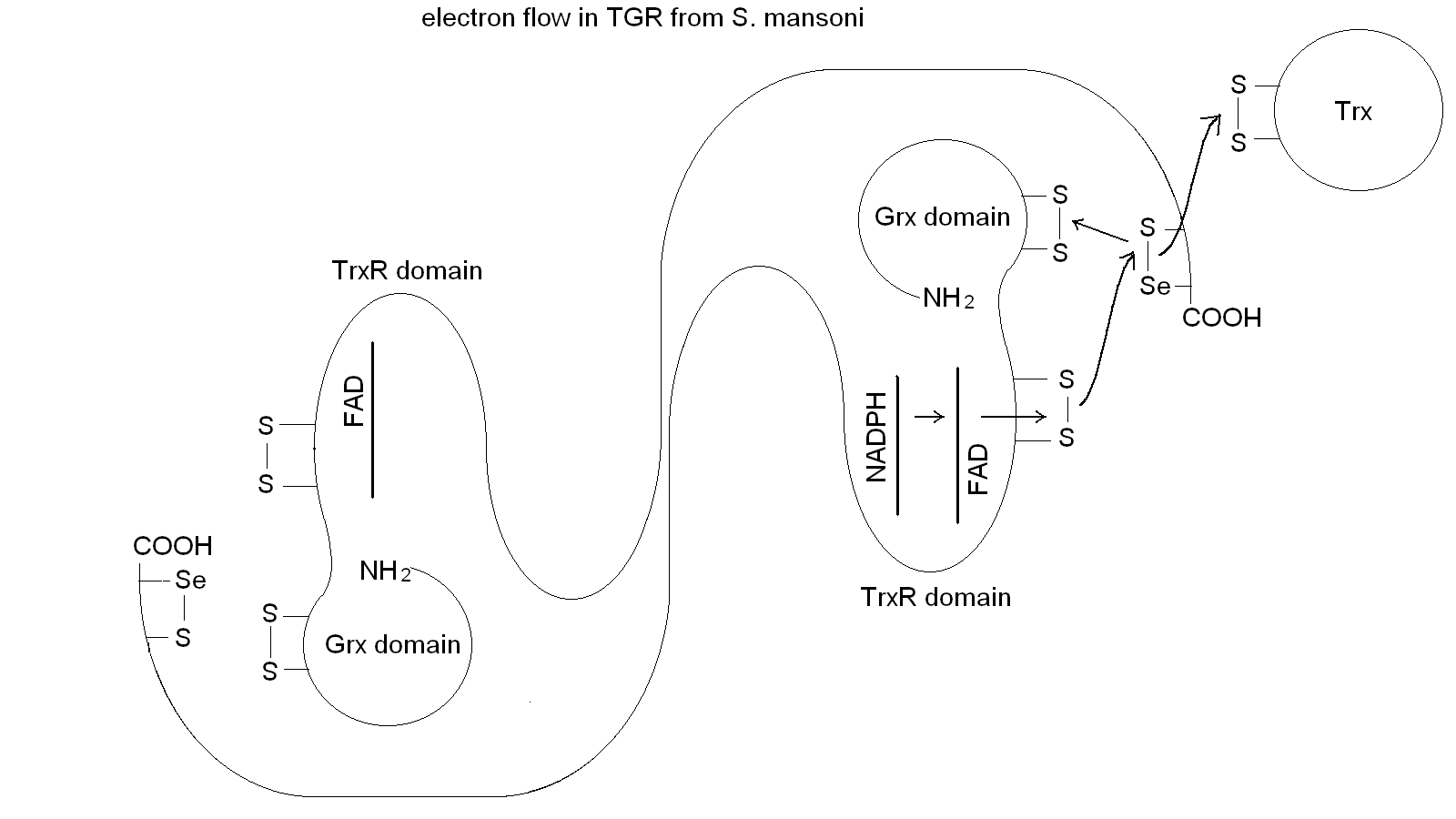
Figura 5: Electron transfer in TGR. Notice the domain swapping 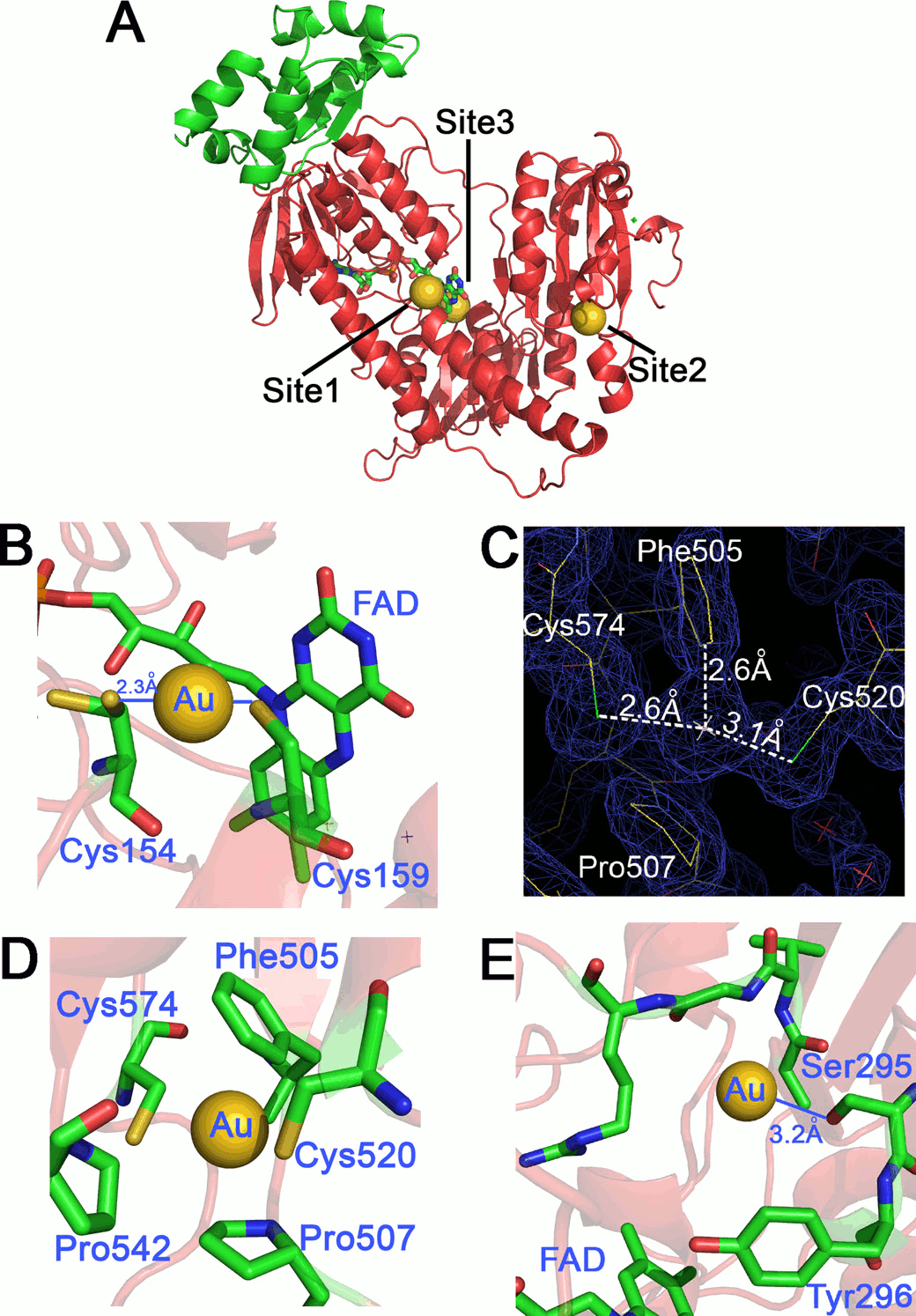
Figura 6: binding sites of gold ions released by auranofin 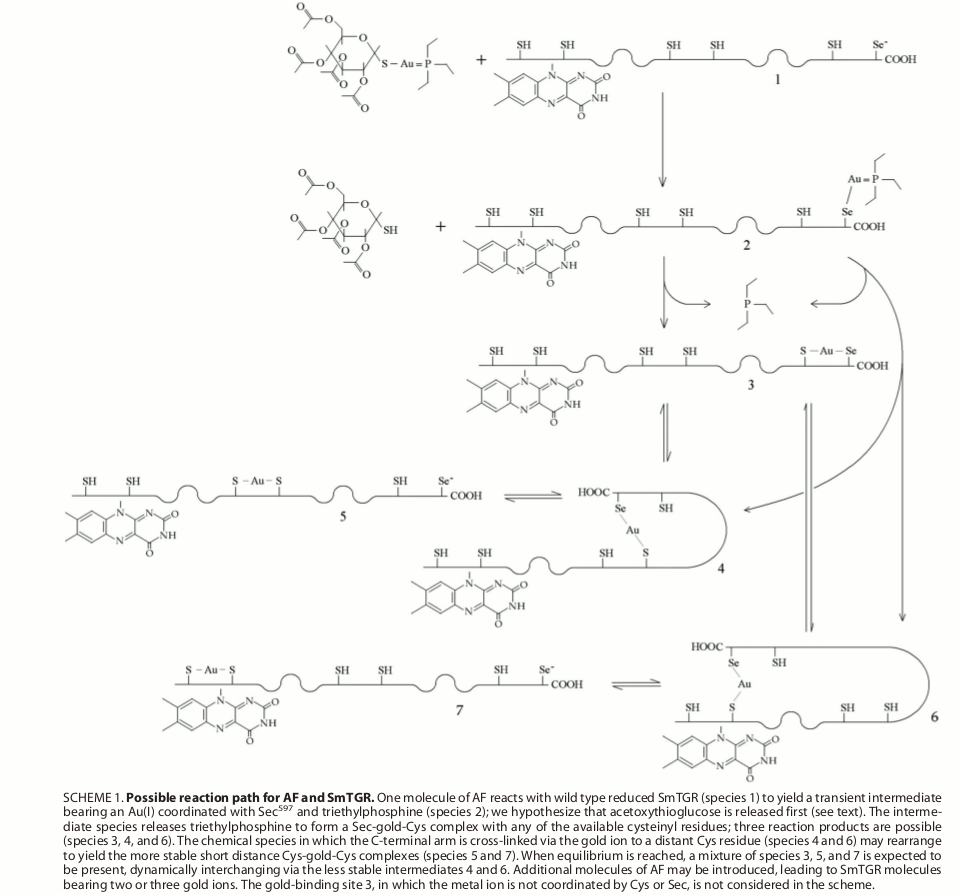
Figura 7: proposed mechanism of transfer of gold ions from auranofin to TGR Selected references Angelucci F, Miele AE, Boumis G, Dimastrogiovanni D, Brunori M, Bellelli A. (2008) Glutathione reductase and thioredoxin reductase at the crossroad: the structure of Schistosoma mansoni thioredoxin glutathione reductase. Proteins; 72: 936-945. Angelucci F, Sayed AA, Williams DL, Boumis G, Brunori M, Dimastrogiovanni D, Miele AE, Pauly F, Bellelli A. (2009) Inhibition of Schistosoma mansoni thioredoxin-glutathione reductase by auranofin: structural and kinetic aspects. J. Biol. Chem.; 284: 28977-28985. Angelucci F, Dimastrogiovanni D, Boumis G, Brunori M, Miele AE, Saccoccia F, Bellelli A. (2010) Mapping the catalytic cycle of Schistosoma mansoni thioredoxin glutathione reductase by X-ray crystallography. J. Biol. Chem.; 285: 32557-32567. Saccoccia F, Angelucci F, Boumis G, Brunori M, Miele AE, Williams DL, Bellelli A. (2012) On the mechanism and rate of gold incorporation into thiol-dependent flavoreductases. J Inorg Biochem.; 108: 105-111. Saccoccia F, Angelucci F, Boumis G, Carotti D, Desiato G, Miele AE, Bellelli A. (2014) Thioredoxin reductase and its inhibitors. Curr. Protein Pept. Sci.; 15: 621-646. |