Course of LABORATORY MEDICINE
Toxicology
A satisfactory classification of toxic substances is made difficult by they great variety. At least four classes of toxic compounds should be considered:
(i) Aspecific toxic compounds. Strong acids and bases and strong oxidizing agents chemically damage several components of the tissues, and especially the cell membranes. The toxic dose is usually elevated.
(ii) Specific toxic compounds. Several toxic substances, either of biological or non-biological origin behave as enzyme inhibitors, agonists or antagonists of receptors, etc. They thus have one or more very specifical and identifiable biological target. Given that these substances act on macromolecules that are present in a limited number of copies in the organism, the toxic dose may be low. Specific poisons may be of inorganic, organic or biological nature: e.g. heavy metals, cyanide, carbon monoxide, mushroom poisons all belong to this group.
(iii) Mutagenic and carcinogenic compounds. Compounds that selectively bind to DNA and interfere with its duplication may cause mutations and cancer. These compounds have an intermediate level of biological specificity with respect to categories (i) and (iii) in that they have a specific macromolecular target (DNA) to which they bind aspecifically (i.e. not to specific genes). As a consequence, mutagenic substances may cause virtually any mutation on any gene.
(iv) Bacterial, plant and animal toxins. These are usually proteins having enzymatic activity against a selected substrate. Poisons belonging to this class have the lowest toxic dose, and it has been calculated that a single molecule of the plant toxin ricin could kill one cell (ricin is an hydrolytic enzyme that removes an adenine base from eukaryotic rRNA, irreversibly inactivating the ribosome). Diagnosis is usually difficult and relies strongly on the anamnesis. Toxins typically target only a few biological systems: e.g. the coagulation system (e.g. several snake poisons are coaugulases), synaptic transmission (e.g. tetanus toxin, an enzyme that digests the protein synaptobrevin), protein synthesis, membrane transporters.
ESSENTIAL CONCEPTS OF PHARMACODYNAMICS
Drugs are administered by several route (e.g. oral, intramuscular, intravenous, etc.) and are transported by the blood to their target organs or cells. The rate of absorption is usually limited by diffusion: the peak blood concentration may be immediate (in the case of intravenous administration) or may be delayed by some hours (in the case of oral administration).
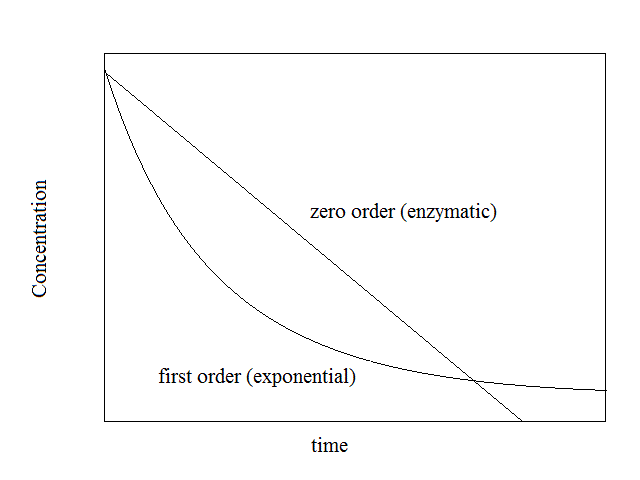
As soon as the drugs appears in the blood the processes of its metabolic transformation and excretion also start. Urinary excretion is usually proportional to the drug concentration in the serum and obeys an exponential (i.e. first order) kinetic law. Enzymatic transformation (generally by the liver followed by excretion via the bile or the urine) is saturable and obeys a zero order kinetic law.
SPECIMEN COLLECTION AND ANALYTICAL METHODS
Each drug has a characteristic therapeutical range (i.e. a range of serum concentration in which it exerts its therapeutic action) and a toxic threshold (the serum concentration at which toxic or undesired effects appear). The therapeutic index is the ratio between the toxic and therapeutical concentrations and is widely different for different drugs: e.g. valium, when used as an ansiolytic is a very safe drug, with therapeutic index >100, whereas lithium ion, only has 2. The dosage, and therefore the therapeutic index of a drug, however, may vary depending on the clinical indications. For example, valium may be used as an antiepileptic drug, ad doses several tens of times higher than those used for its ansiolytic activity. In these cases its therapeutic index is substantially lower.
Accidental drug intoxications are common especially in the elderly and in children, and require prompt diagnosis and treatment. The anamnesis is crucial to identify the drugs that were available to the patient.
Determination of the serum (or other biological fluid) concentration of a drug is indicated if: (i) the therapeutc index is low; (ii) absorption or excretion exhibit large interindividual variability (especially in the presence of liver or kidney diseases); (iii) non-compliance or abuse by the patient are suspected; (iv) unexpected toxic effects appear.
The appropriate sample is usually the serum; in some cases the urine,bile or other body fluids. In some cases the drug concentration is effectively measured; in other cases a characteristic metabolyte is measured instead.
Analytical methods typically used in clinical toxicology include: chromatography (e.g. high pressure liquid chromatography; gas chromatography); potentiometry; spectrophotometry; mass spectrometry; radio-immunoassay and ther immunological methods.
SOME DRUGS THAT MOST COMMONLY REQUIRE TOXICOLOGICAL ANALYSES
Some drug categories require toxicological determinations, because of essentially two main reasons: (i) the therapeutic index is low, thus even small deviations from mean values may cause risk; or (ii) the pharmacodynamics exibits high inter-individual variability.
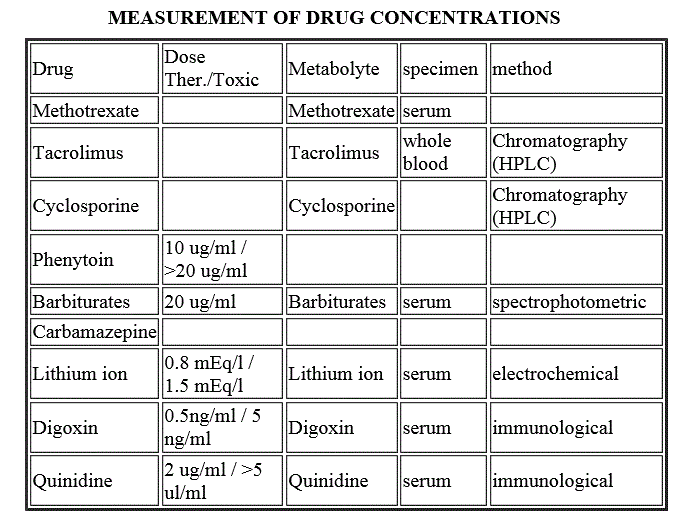
MEASUREMENT OF DRUG CONCENTRATIONS
| Drug | Dose Ther./Toxic | Metabolyte | specimen | method |
| Methotrexate | Methotrexate | serum | ||
| Tacrolimus | Tacrolimus | whole blood | Chromatography (HPLC) | |
| Cyclosporine | Cyclosporine | Chromatography (HPLC) | ||
| Phenytoin | 10 ug/ml / >20 ug/ml | |||
| Barbiturates | 20 ug/ml | Barbiturates | serum | spectrophotometric |
| Carbamazepine | ||||
| Lithium ion | 0.8 mEq/l / 1.5 mEq/l | Lithium ion | serum | electrochemical |
| Digoxin | 0.5ng/ml / 5 ng/ml | Digoxin | serum | immunological |
| Quinidine | 10-20 ug/ml / >5 ul/ml | Quinidine | serum | immunological |
| Theophylline | 2 ug/ml / >30 ul/ml | Theophylline | serum | immunological |
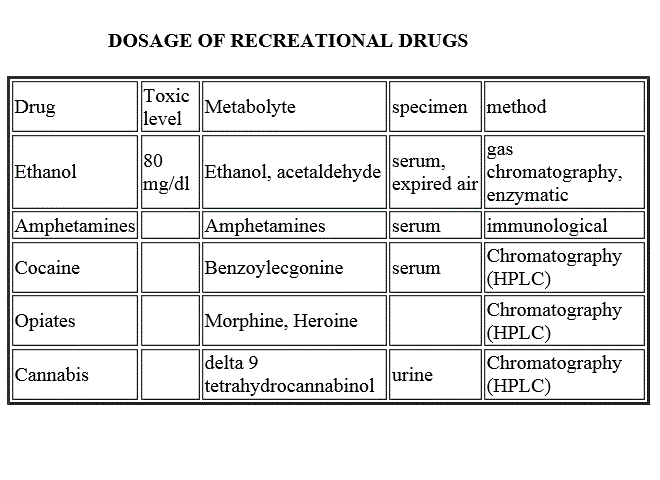
RECREATIONAL DRUGS
Recreational drugs are taken by the patient in the absence of a physician's prescriptions and without therapeutical scope. Most of them are toxic to the central nervous system, and the patient may be brought to the emergency room, often in an unconscious state. As a consequence anamnestic information may not be available, and one has to guess whether a drug was used and which one. Detection of the drug used by the patient is essential for an appropriate therapy, and has forensic relevance. The following table lists some commonly used recreational drugs.
| Drug | Toxic level | Metabolyte | specimen | method |
| Ethanol | 80 mg/dl | Ethanol, acetaldehyde | serum, expired air | gas chromatography, enzymatic |
| Amphetamines | Amphetamines | serum | immunological | |
| Cocaine | Benzoylecgonine | serum | Chromatography (HPLC) | |
| Opiates | Morphine, Heroine | urine, serum | Chromatography (HPLC; gas chromatography) | |
| Lysergic acid (LSD) | LS Diethylamide; 2-oxo 3-hydroxy LSD | urine, serum | Chromatography (HPLC) | |
| Cannabis | delta 9 tetrahydrocannabinol | urine | Chromatography (HPLC) |
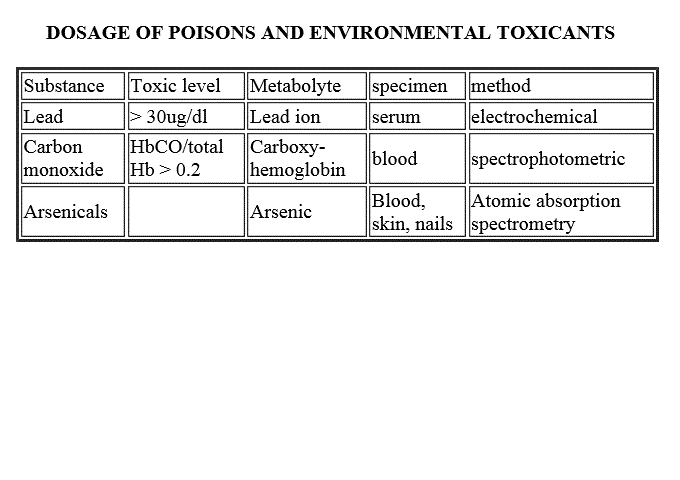
DOSAGE OF POISONS AND ENVIRONMENTAL INTOXICANTS
Acute or chronic intoxications occur frequently because of several reasons: some commonly used detergents and bleaching agents are highly toxic (e.g. ipochlorite, common bleach), or may cause chemical burns (e.g. sodium hydroxide, hydrochloric acid, permanganate); some jobs entail risks of professional poisoning (e.g. miners, workers of chemical factories, etc.); food may be contaminated with pesticides and other compounds used in agriculture, or polutants; etc. In the following list only some of the most common poisons are included.
| Substance | Toxic level | Metabolyte | specimen | method |
| Lead | > 30ug/dl | Lead ion | serum | electrochemical; atomic absorption spectrometry |
| Mercury | > 0.6ug/dl | Mercury | (serum) urine, tissues | electrochemical; atomic absorption spectrometry |
| Carbon monoxide | HbCO/total Hb > 0.2 | Carboxy-hemoglobin | blood | spectrophotometric |
| Arsenicals | Arsenic | Blood, skin, nails | Atomic absorption spectrometry | |
| Organophosphates | Blood, urine | detection of organophosphate metabolytes by chromatography; reduced esterase activity |
Lead poisoning
Lead poisoning is a relatively common cronic (more rarely acute) professional disease. The disease affects several organs and tissues. Neurological symptoms are common, with insomnia, tremor and cognitive defects. Peripheral neuropathy may be responsible of painful crisis, usually referred to the abdomen (so called saturnine colic, often misdiagnosed as appendicitis). Anemia is frequent, as is kidney failure. In symptomatic cases lead is presnt in the blood at concentration > 30-40 ug/dL. The ion is measured in the blood, tissues and urine by means of potentiometry or atomic absorption spectroscopy. Chelation therapy is indicated.
Other toxic metals
Essentially every metal ion if absorbed in excess may cause toxicity. Some intoxications are uncommon: e.g. sodium, potassium, calcium, and magnesium are physiologically present at high concentration in our body and we have effective excretion routes, thus intoxication can only occur because of parenteral administration. Iron is physiologically present in our body and has no physiological excretion pathway: we loose iron because of hemorrages. However, the absorption of iron is strictly regulated, thus we do not risk iron intoxication except in two cases: (i) because of inherited defects in the regulation of absorption (primary hemochromatosis); or (ii) because of parenteral administation, usually in the form of blood transfusion (secondary hemocromatosis). Chelation therapy is indicated.
Mercury is a highly toxic metal, that may be absorbed from the environment where it is present as a free metal or in the form of its ogranometallic derivative (e.g. methylmercuric chloride). It reacts with Cys residues of enzymes and blocks their action. Acute mercury poisoning leads to kidney insufficiency and death. Early and specific chelation therapy is highly recommended (the chelator of choice is di-mercapto propanol). Mercury can be detected in the blood or in any tissue by means of atomic absorption spectroscopy.
Cadmium, chromium and other transition metals may be responsible of human poisoning, usually in workers of specialized factories (e.g. varnish). Diagnosis is established by atomic absorption spectroscopy.
Arsenic is a special case because it is a metalloid and presents metallic and non-metallic behaviour in different compounds. Toxicity depends on the arsenic compound that has been absorbed. The atom itself can form complexes with sulfur and may inhibit enzymes whose activity requires a Cys residue. Arsenic, and in particular the arsenite ion (AsO3
Organophosphate and organochlorine pesticides
Organophosphoric compounds are selective covalent inhibitors of Ser- and Tyr- esterases, most typically of acetyl cholinesterase. These compounds are widely employed in agriculture as pesticides and herbicides, thus professional or accidental poisoning is common. Some compounds of this class have been used as tosic gases for chemical warfare or terroristic attacks. The symptoms of acute organophosphate poisoning is the cholinergic crisis, due to excess activity of the cholinergic (parasympatic) system: convulsions, ataxia, depression of respiration and circulation, tremor, general weakness, possibly coma and death. Diagnosis relies on two tests: (i) detection of the metabolytes of organophosphates in the blood and urine, by means of liquid chromatography; and (ii) measurement of reduced pseudocholinesterase activity in the serum.
Organochlorine pesticides (e.g. DDT) are banned in Europe and USA, but have been widely employed in the past and have long persistence in the environment. THese compounds target the peripheral nervous system, acting as agonists of sodium channels or as antagonists of chloride channels. They have significant toxicity for mammals, and may cause liver insufficiency and reduced fertility.
Home of this course
Slides of this lecture:
 |
 |
 |
 |
 |
 |
 |
 |