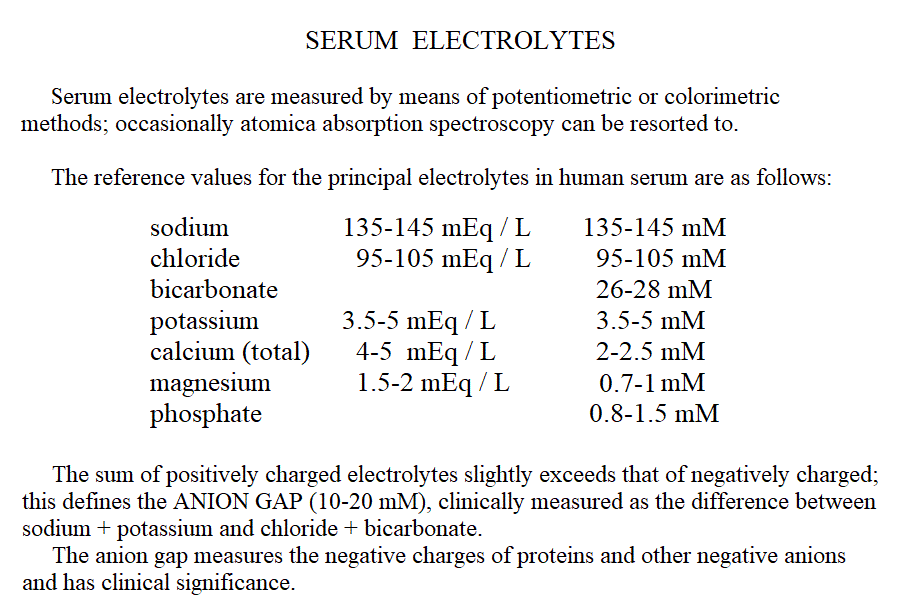
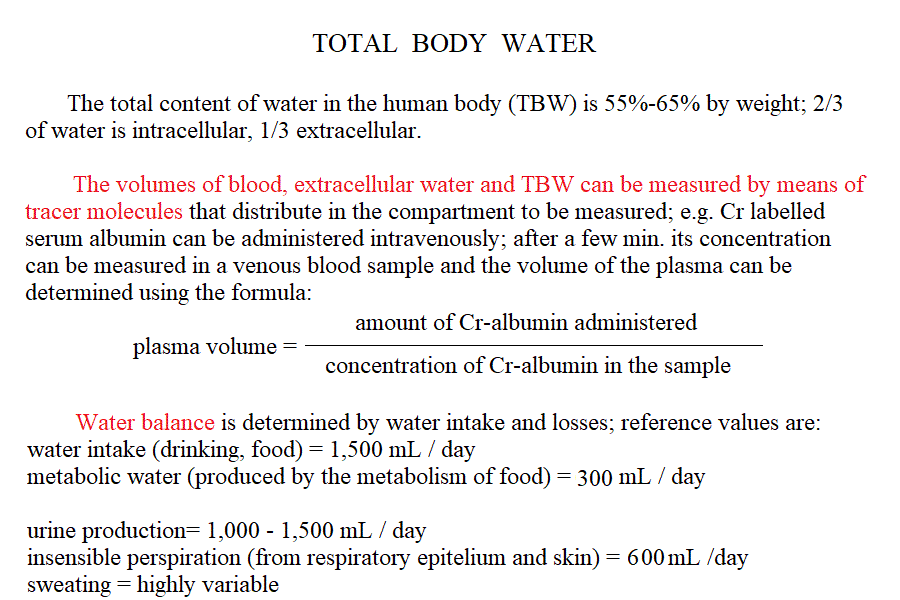
| Course of LABORATORY MEDICINE Disturbances of water, pH and electrolyte balance Water and electrolytes are subject to a rapid turnover due mainly to dietary intake and renal excretion. Other causes of water (and electrolytes) elimination are faeces, respiration and sweating. Under pathological conditions water and electrolyte loss may occur because of several reasons that include: diarrhoea (e.g. cholera, bacterial enteritis or cholitis); vomiting, inability of the kidney to concentrate the urine (e.g. diabetes mellitus, diabetes insipidus). As a general rule, water is absorbed or excreted passively, thus it follows the main extracellular electrolytes, sodium and chloride, and water deficits usually occur together with sodium and chloride deficits. It is important to remark, however, that the intracellular water compartment is usually most affected and that symptoms may occur also under conditions in which the water and electrolyte deficits one may estimate from blood composition are relatively mild. The central nervous system is particularly sensitive to water and electrolyte unbalance and symptoms (e.g. irritability, convulsions, coma) may be severe.
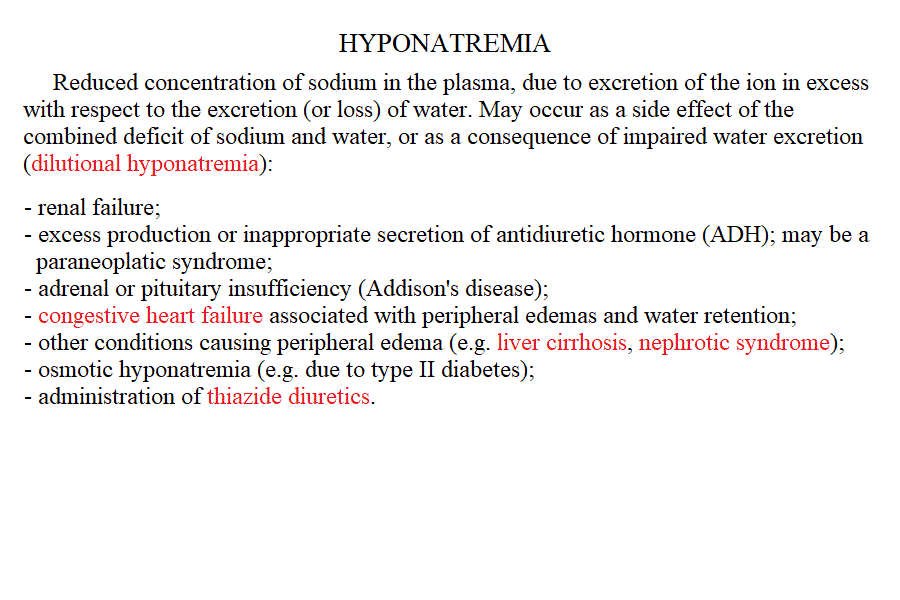
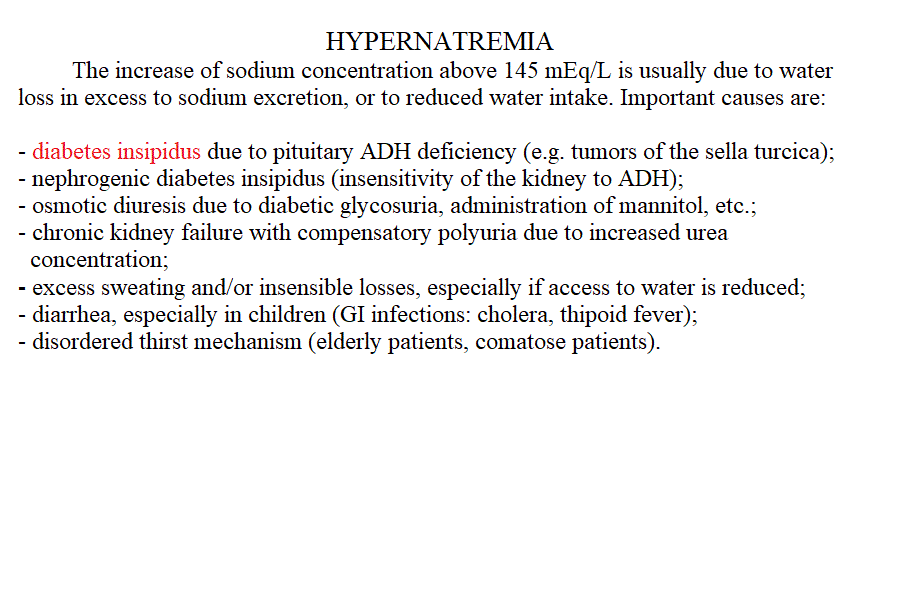
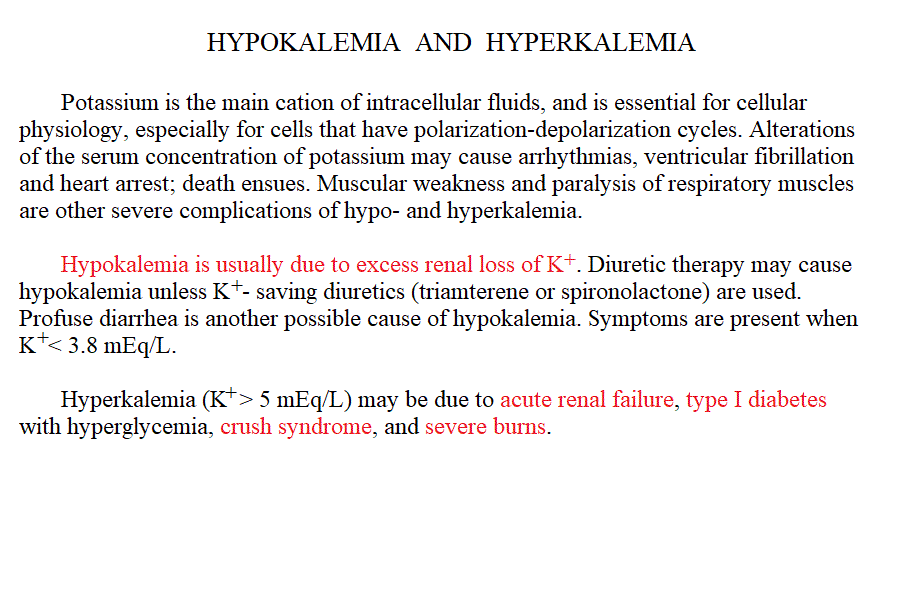
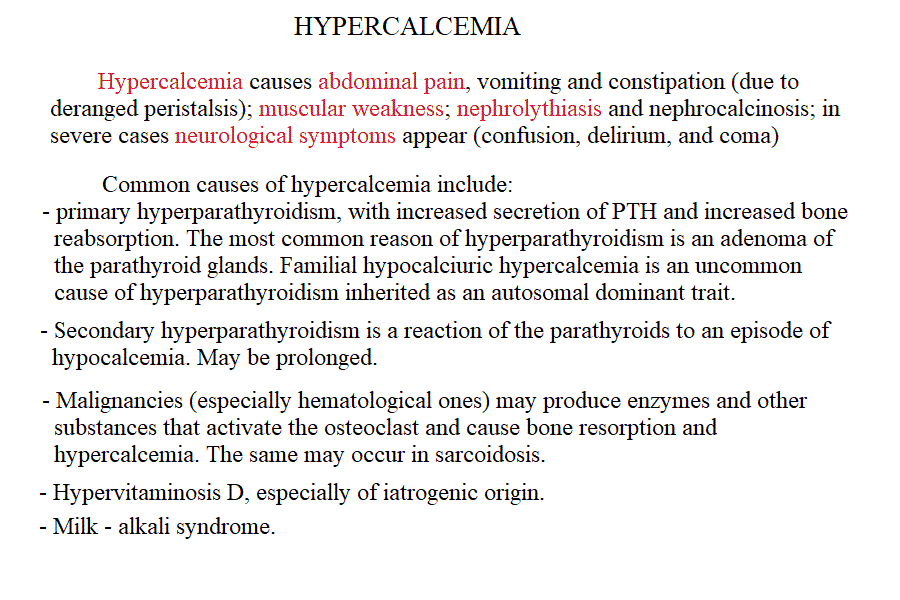
Measurement of electrolyte concentration in the blood is usually effected by potentiometric methods, using the appropriate electrodes. Other methods are less commonly employed, e.g. flame atomic absorption. Hyponatremia is a condition in which sodium losses exceed water losses; decrease of the electrolyte concentrations and hypo-osmolarity follow. This condition may occur in several types of renal failure, in heart failure because of the sodium retention in peripheral oedema, in several endocrine disease and in neoplastic diseases, in the course of diuretic therapy. An uncommon but important cause is the (usually paraneoplastic) syndrome of inappropriate secretion of antidiuretic hormone (ADH). Water intoxication occurs when the plasma osmolarity falls belo 240 mOsm/Kg (normal value approx 300 mOsm / Kg). Hypernatremia (i.e. increased concentration of serum electrolytes, especially sodium) is typically due to water deprivation or increased and unreplaced water losses (e.g. profuse sweating). It requires prompt rehydration, in severe cases by intravenous administration of glucose isotonic solution. Hypokalemia is due to excessive loss of potassium in the urine, faeces or sweat. Renal waste of potassium occcurs in Bartter syndrome (a disease of unknown origin) and in the presence of excess secretion of mineralocorticoid hormones (e.g. because of a benign tumour of the adrenal glands) and in primary disturbances of the kidney involving the proximal and distal tubuli. It may be iatrogenic, due to excess diuretic therapy (e.g. using thiazides or furosemide) and it is always advisable in prolonged diurteic therapy to associate a K-saving diuretic (e.g. spironolactone). Normal potassiemia is 5 mEq/L and in severe depletion (3 mEq/L or less) muscular weakness, and severe cardiac arrythmias, both due to the fact that excitable tissues function is compromised if the intra- extra-cellular potassium gradient is altered. Severe hypokalemia requires (careful !) intravenus administration of potassium. Hyperkalemia may occur in type I diabetic patients or in the course of severe acute kidney disease (e.g. glomerulonephritis, acute renal failure), given that the kidney excretes potassium very efficiently and, if functional prevents or corrects this condition. Pseudohyperkalemia is the transient increase of potassium concentration due to release of intracellular potassium by the red or white blood cells or by platelets. True hyperkalemia is a dangerous condition that requires prompt treatment, given that at potassiemia > 6.5 mEq/L severe arrythmias occur and ventricular fibrillation is possible. Disturbances of Calcium metabolism. The concentration of calcium in the blood serum in the healthy adult is approx. 2.5 mMoles/L (or 5 mEq/L or 10 mg/dL) and is regulated by two hormones and one vitamin: the Parathyroid hormone (PTH) is a protein secreted by the parathyroid glands; it causes reabsorption of calcium from the skeletal deposits, from the urine and from the gut and causes the calcemia to increase. Thyrocalcitonin is a protein hormone produced by th eparafollicular cells of the thyroid and causes calcium phophate deposition in the bone matrix, opposing the effect of PTH. Vitamin D promotes absorption of calcium from the diet. The serum concentration of calcium is so carefully regulated because this ion is essential for the excitability of nervous and muscular tissues and changes in its concentration cause severe symptoms. Hypocalcemia is an infrequent finding; it may depend on several causes, among which: (i) hypoparathyroidism (often associated to surgical removal of the parathyroid in the course of a thyroidectomy); (ii) vitamin D deficiency (e.g. rickets); (iii) renal tubular disease or renal failure; (iv) acute pancreatitis (calcium chelation by lipolytic products). Clinical symptoms include reduced cerebral function (pseudo-dementia), possibly with psychotic symptoms, and muscular tetany. Hypercalcemia is usually caused by hyperparathyroidism (often of neoplastic origin), and is associated to excessive bone matrix reabsorption (osteoporosis). Other neoplastic diseases, unrelated to the parathyroids, can cause osteolysis with hypercalcemia and osteoporosis, because of secretion of osteoclast activating factors (so called "humoral hypercalcemia of malignancy"). Hypervitaminosis D is another possible cause of hypercalcemia. Hypophosphatemia. Calcium phosphate is the main mineral component of the bone tissue, and mobilization of phosphate usually follows that of calcium: e.g. PTH causes reabsorption of both calcium and phosphate. However, in the intracellular milieu and in the diet calcium and phosphate have different distributions and thus changes in the serum concentrations of phosphate does not necessarily follow those of calcium. Hypophosphatemia is not uncommon but rarely severe or even symptomatic. The main cause is reduced renal reabsorption. Disturbances of Magnesium metabolism. In the healthy adult the serum magnesium concentration is approx. 2 mEq/L and is regulated mainly at the level of urinary and fecal excretion. Hypomagnesemia may result from prolonged poor dietary intake or reduced intestinal absorption (diarrhoea, malnutrition, etc.) it may be aggravated by some physiological conditions of excess consumption (e.g. lactation). Hypermagnesemia may occur, together with other electrolyte disturbances, in chronic or acute renal failure. The alterations of the blood pH are called acidoses (if arterial pH < 7.35) and alkaloses (if arterial pH > 7.45). They are due to abnormal production or excretion of acidic or basic solutes in the serum, and of course are counteracted by the blood buffers. An interesting and well-written tutorial may be found at this link; a very useful article on the subject may be read at this link.
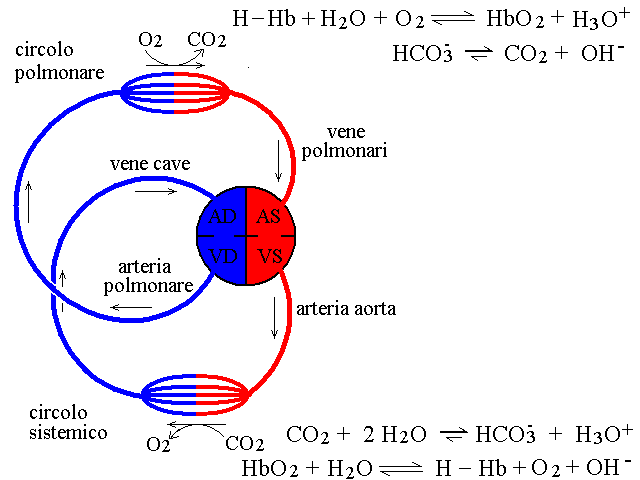
Even though the blood buffers behave in quite a complicated manner, over the physiological range their titration curve can be roughly approximated to that of a single weak acid with pK approx. 6.5. The lung and the kidney are the principal organs involved in the regulation of buffer concentration and blood pH, given that they excrete CO2 and bicarbonate, the components of the principal buffer (and the kidney excretes phosphate as well). The lung alone eliminates some 20 moles CO2 / day, a massive amount. A simplified scheme of the gas exchanges in relation to blood pH is depicted in teh following figure. 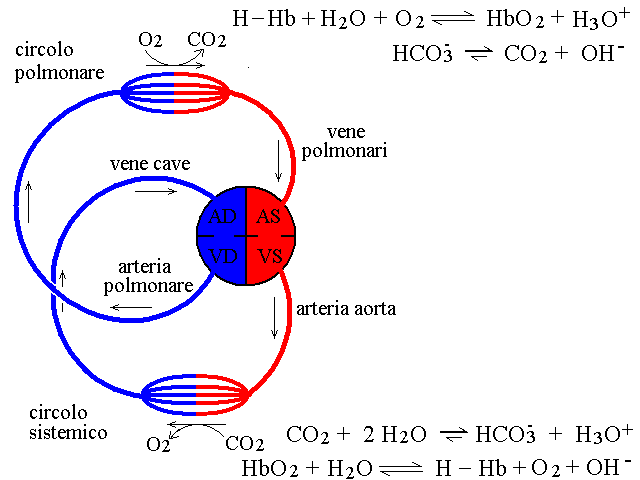 The most concentrated buffer of blood is bicarbonate; the Henderson Hasselbalch equation for this buffer is as follows Disturbances of the blood pH and buffer concentrations are called respiratory if caused by altered functioning of the lungs, metabolic otherwise. It is important to stress that disfunction of the lung can be to some extent compensated by the kidney and vice versa; thus each organ tends to oppose the disfunction of the other. The key features of the different forms of acidosis and alkalosis are as follows:
The following remarks will help explaining the above table: (i) Pressure of blood CO2 indicates the concentration of pure CO2, i.e. it does not include bicarbonate; total CO2 is dominated essentially by bicarbonate ion concentration (the ratio [HCO3-] / [CO2] being approximately 20). (ii) The metabolism produces mainly acids (CO2 and lactic acid, acetoacetic acid, etc.): thus acidosis is more frequent, more severe and more varied; alkalosis is often caused by excess loss of acidic fluids (e.g. severe vomiting). (iii) Acids can be volatile (CO2), excreted by the lung at a very fast rate, and non-volatile (lactic acid, acetoacetic acid), excreted mainly by the kidney at a slower rate. (iv) Metabolic alkalosis is most often due to loss of acids (e.g. vomiting), excess intake of alkaline substances (e.g. gastric antiacids, bicarbonate), and diuretics; it is corrected mainly by the kidney that excretes the excess bicarbonate. (v) Metabolic acidosis, caused by overproduction of non-volatile acids (e.g. diabetic ketoacidosis) or by their impaired renal excretion, stimulates respiration that excretes the volatile acid (CO2): hence compensatory hyperpnea and hypocapnia (reduced P CO2). It is interesting to remark that pulmonary correction of metabolic acidosis is more effective than of metabolic alkalosis because respiration frequency can be increased to a more significant extent than it can be decreased. (vi) Respiratory alkalosis is a consequence of hyperventilation (loss of CO2), but this only occurs in some types of CNS disturbances or under unusual environmental conditions (e.g. muscular effort at high altitude, where atmospheric P O2 is decreased - air hunger). (vii) Respiratory acidosis is a common consequence of impaired gas exchanges (e.g. depression of respiratory centers in the CNS, insufficient mechanical ventilation in polyomyelitis or tuberculosis, or ventilation perfusion imbalance in chronic obstructive pulmonary disease, emphysema, etc.). The hemogas analysis is the measurement of the pH and the concentrations and partial pressures of O2 and CO2 in a sample of the patient's blood drawn in a gas tight syringe. The measure is usually effected by means of potentiometric methods, used gas-specific electrodes. As a general rule, a hemogas analysis will indicate if an abnormality is present and will give some indication of its possible cause; the fundamental indications are as follows: Diminished pH and diminished total CO2 = acute or chronic metabolic acidosis with respiratory compensation (e.g. diabetic ketoacidosis). Strongly diminished pH, strongly increased PCO2 and slightly increased total CO2 = acute respiratory acidosis. The exchange of CO2 in the lung is impaired (e.g. because of acute viral pneumonia); this leads to increased arterial PCO2 and decreased arterial pH. Metabolic compensation is scarce or absent because it requires several days to become operative; this causes bicarbonate and total CO2 to increase only slightly. Normal to diminished pH and strongly increased total CO2 = chronic respiratory acidosis. If the impaired gas exchange in the lung lasts long enough for the kidney to retain bicarbonate, metabolic compensation occurs (e.g. chronic obstructive pulmonary disease). Retention of bicarbonate (partially) restores the arterial pH but causes a strong increase in bicarbonate concentration and total CO2. Increased pH and increased total CO2 = metabolic alkalosis with respiratory compensation (e.g. vomiting). Increased pH and decreased PCO2 with moderately increased bicarbonate = acute respiratory alkalosis with minimal metabolic compensation (uncommon; e.g. neurological hyperpnea). Normal to increased pH and decreased total CO2 = chronic respiratory alkalosis with metabolic compensation (e.g. life at high altitude). The above set of rules allows one to interpret simple deviations from the healthy conditions, i.e. those conditions where one disease (either respiratory or metabolic) is present and compensation is respiratory. These conditions are typical of young patients suffering of acute acid-base imbalance (notice that respiratory compensation of metabolic conditions is almost immediate, whereas metabolic compensation requires time). Notice that two parameters (pH and total CO2) are necessary even in the least complicated cases. The anion gap, is the difference between the concentrations of (sodium + potassium) and (chloride + bicarbonate). The blood, as any other mixture, has zero net charge; thus the anion gap estimates the amount of non-measured negative charges (e.g. proteins). The normal value is 10-15 mM. An increased anion gap may provide a gross indication of the presence of excess unmeasured negatively charged ions derived from carboxylic acids (e.g. lactate or acetoacetate). Accordingly there are two types of metabolic acidosis, either with normal anion gap (NAGMA: Normal Anion Gap Metabolic Acidosis) or with increased anion gap (HAGMA: High Anion Gap Metabolic Acidosis).
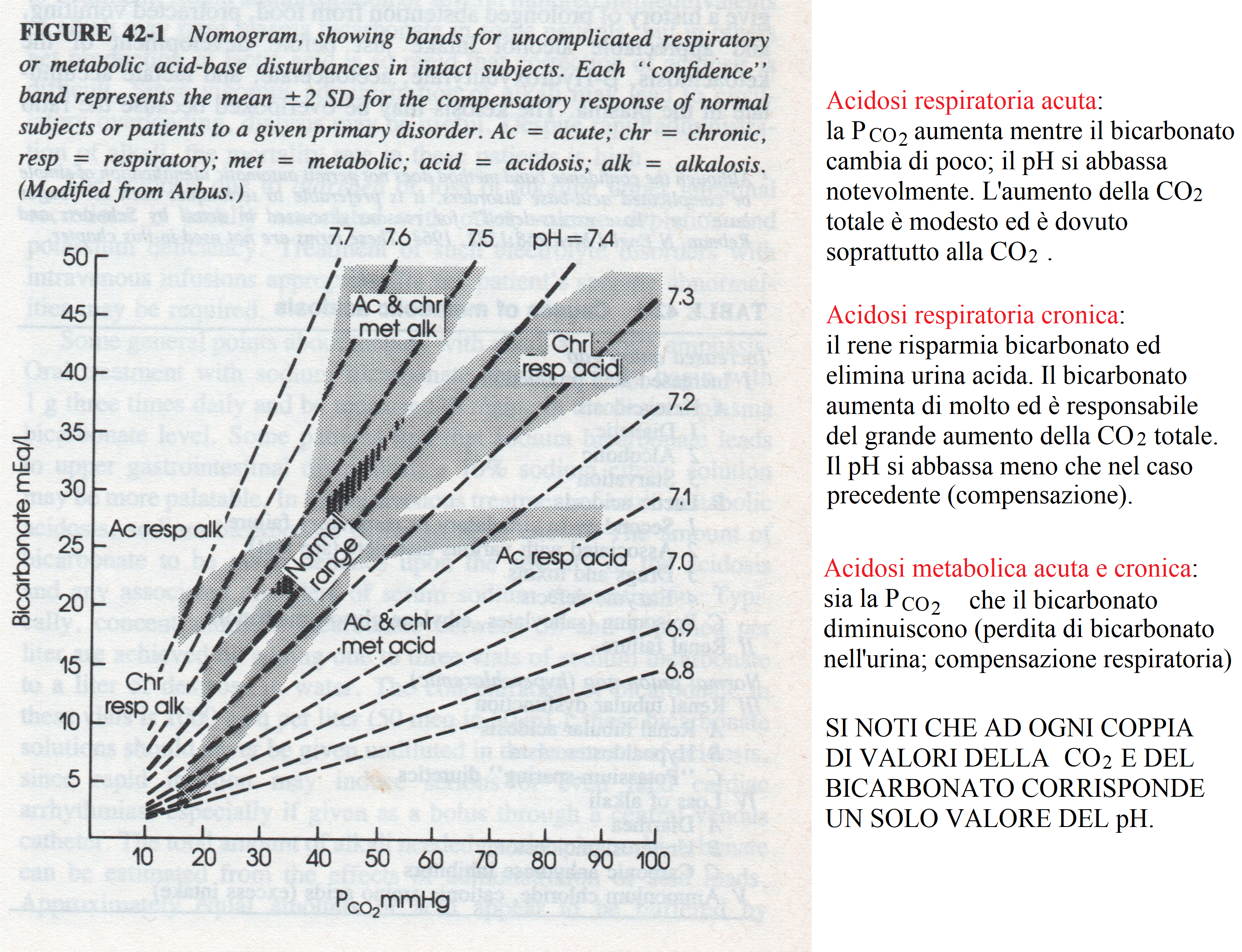
A very useful representation of the results of a hemogas analysis is a plot of bicarbonate concentration versus PCO2. Because of the Henderson-Hasselbalch equation each couple of these parameters identifies a pH value, and couples corresponding to the same pH appears as lines in this graph. 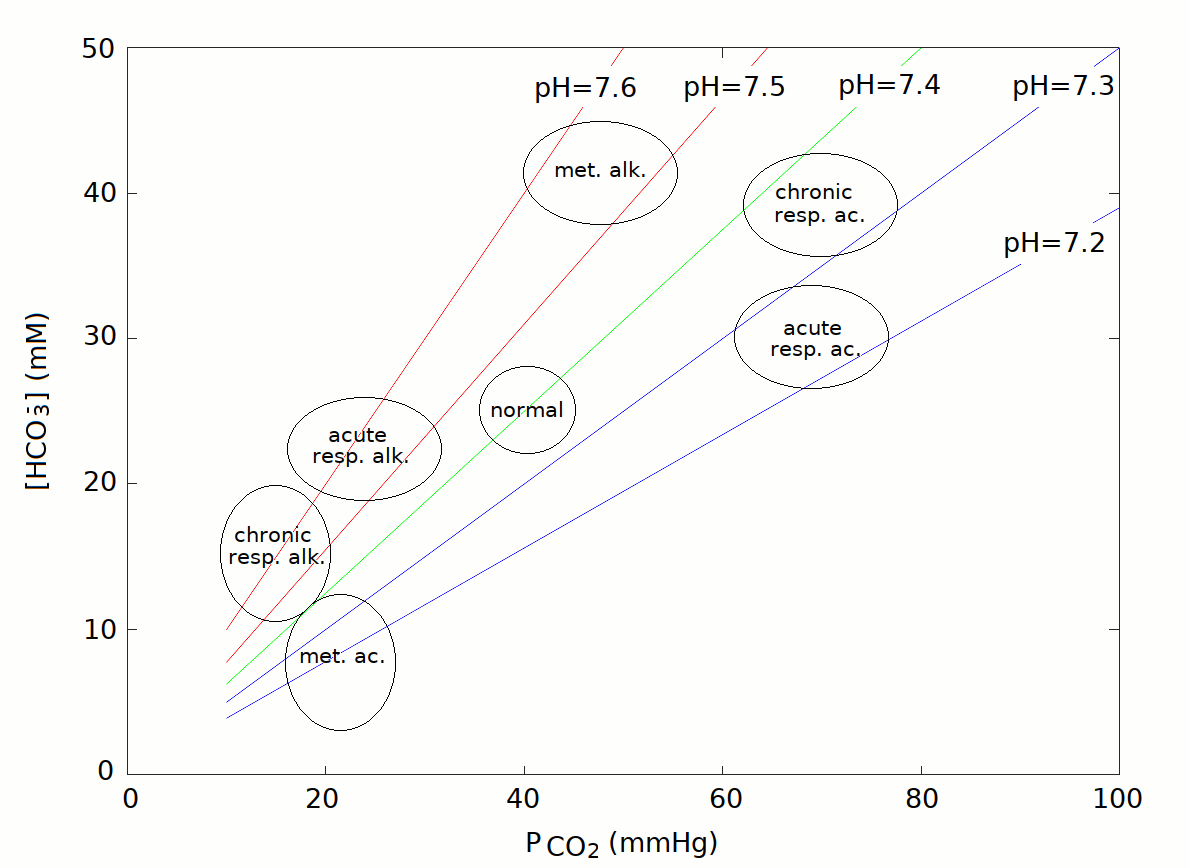
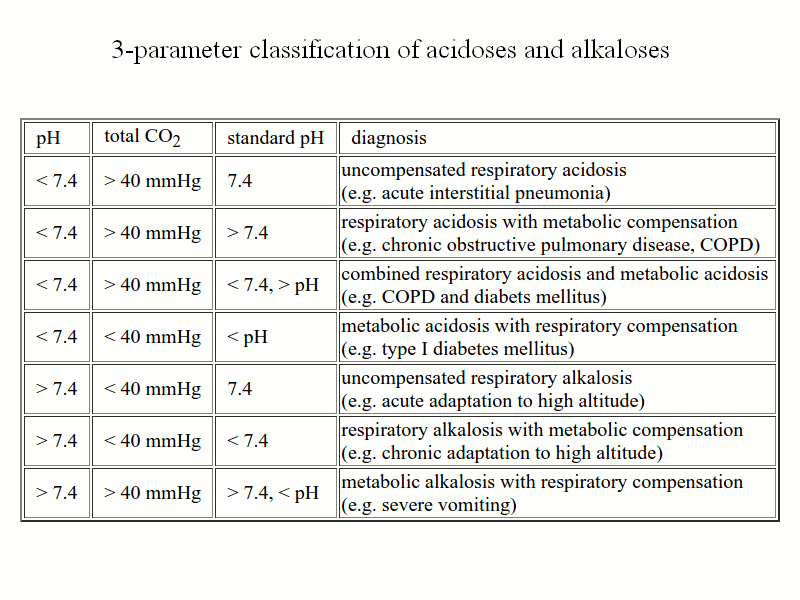
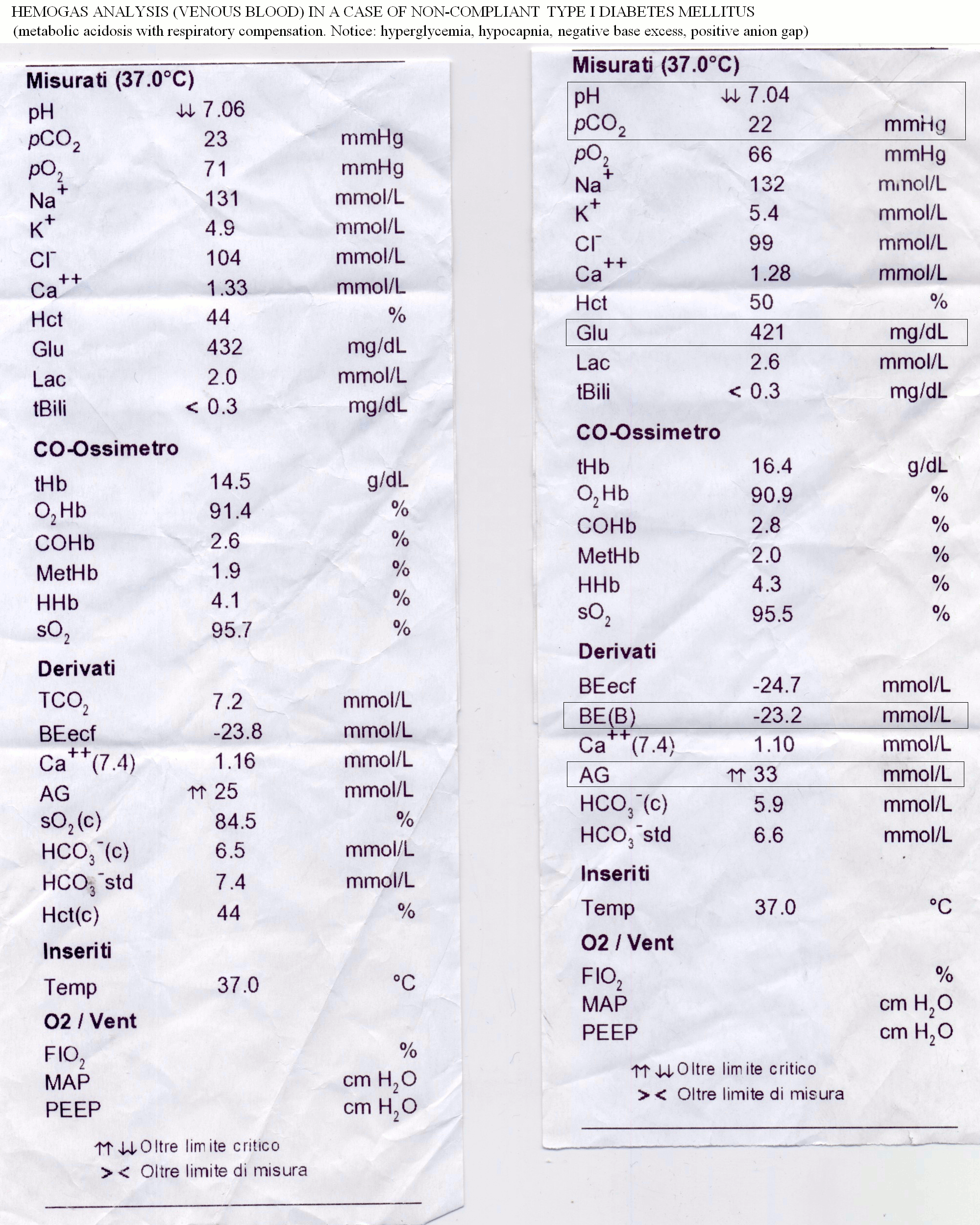
The graph identifies regions of the (PCO2,[HCO3-]) space corresponding to the main categories listed above: acute respiratory acidosis and alkalosis, chronic respiratory acidosis and alkalosis, metabolic acidosis and alkalosis. The main reason why respiratory conditions require the distinction between acute and chronic is that renal compensation requires several days, thus acute respiratory conditions are poorly compensated or uncompensated, whereas chronic respiratory conditions are usually well compensated. Metabolic conditions require respiratory compensation, which is established rapidly: thus they are usually well compensated irrespective of their onset being acute or chronic. We remark that chronic, compensated respiratory conditions may be compatible with a normal blood pH of 7.4, but are revealed by grossly altered values of PCO2 and bicarbonate. Basic 4-parameter diagnostic interpretation of the arterial hemogas analysis. 1) The first parameter to analyze is the blood's pH. This defines the conditions of acidosis and alkalosis, even though in the presence of effective compensation the alteration of the blood's pH may be minimal or even absent. 2) PCO2 is the second most important parameter, which distinguishes hypocapnic from hypercapnic conditions. The general rule for interpreting this parameter in conjunction with the blood's pH is reported in the above Table "Types of acidosis and alkalosis". Since the PCO2 is primarily controlled by respiration, important changes of this parameter reflect either pulmonary disease or respiratory compensation. Even though PCO2 and bicarbonate are correlated by the henderson and Hasselbalch equation, changes in PCO2 due to pulmonary disease affect the pH more strongly than the bicarbonate concentration, unless metabolic compensation intervenes. 3) Total CO2 is essentially determined by bicarbonate concentration. Bicarbonate is primarily controlled by the kidney and and important changes of its concentration reflect metabolic disease or compensation. 4) The anion gap is required to distinguish HAGMA from NAGMA. Some typical examples. 1) Chronic obstructive pulmonary disease (COPD), diffuse interstitial pneumonia, etc. reduce the efficiency of gas exchanges. A typical hemogas analysis may be as follows: oxygen saturation 78% on room air (normal value > 90%) arterial pH 7.25 (normal value 7.44) PCO2 70 mmHg (normal value 40-44 mmHg) PO2 50 mmHg (normal value > 80 mmHg) plasma bicarbonate concentration 35 mM (normal value 26 mM) Anion gap 12 mEq/L 4-parameters analysis of this case is as follows: 1) pH is decreased, thus this condition is an acidosis. 2) PCO2 is significantly increased, thus this condition is a respiratory acidosis. 3) Bicarbonate and total CO2 are increased, thus metabolic compensation is present. Since metabolic (renal) compensation requires several days, this condition is a chronic respiratory acidosis. 4) The anion gap is normal; this occurs because the increase in bicarbonate is associated to renal excretion of chloride. Description: chronic hypercapnic acidosis, associated (in this case) to reduced oxygen content and oxygen saturation. 2) Adaptation to high altitude (4.000 m above sea level or higher). A typical hemogas analysis may be as follows: oxygen saturation 75% on room air arterial pH 7.48 PCO2 20 mmHg PO2 60 mmHg plasma bicarbonate concentration 16 mM Anion gap 13 mEq/L 4-parameters analysis of this case is as follows: 1) pH is increased, thus this condition is an alkalosis. 2) PCO2 is significantly decreased, thus this condition is a respiratory alkalosis. 3 and 4) Bicarbonate and total CO2 are increased, thus metabolic compensation is present. The normal anion gap is normal because increased urinary excretion of bicarbonate is associated to chloride retention. Diagnosis: chronic respiratory hypocapnic alkalosis due to hyperpnea (this is an attempt to compensate for the reduced atmospheric PO2) 3) Type I diabetes mellitus: plasma glucose > 250 mg/dL arterial pH < 7.25 PCO2 20 mmHg PO2 normal serum bicarbonate 10-20 mM anion gap 25 mM 4-parameters analysis of this case is as follows: 1) pH is decreased, thus this condition is an acidosis. 2 and 3) PCO2 and bicarbonate are both significantly decreased, thus this condition is a metabolic acidosis with respiratory compensation. 4) The anion gap is increased, i.e. the plasma contains an excess of non-measured anions: HAGMA. Diagnosis: the most important causes of HAGMA are:renal failure, ketoacidosis, lactic acidosis, inherited defects of metabolism (e.g. methylmalonic aciduria), and several types of poisoning. In the present case the increased glycemia suggests diabetic metabolic acidosis due to ketone bodies (acetoacetic acid and 3-hydroxy butanoic acid). The anion gap is increased because of the presence of the non-measured anions acetoacetate and 3-hydroxy butanoate. More complex cases require additional parameters. In the presence of an acid-base imbalance whose diagnosis is not obvious, more refined measurements are indicated in order to separate the respiratory and metabolic cotributions. Special attention is required in the elderly given that metabolic and respiratory conditions of equal or opposite sign may coexist (e.g. pulmonary emphysema, causing chronic respiratory acidosis, may be present together with vomiting, causing acute metabolic alkalosis, or with diabetes, causing chronic metabolic acidosis). Several clinical concepts (and measurements) have been developed to discriminate the metabolic and respiratory components of blood buffers inbalance, as listed below: Standard pH, historically the first concept introduced to rationalize complex deviations from the healthy conditions of blood buffers balance was introduced into the clinical practice by Hasselbalch in 1916. It is the pH of the patient's arterial blood measured under standard conditions (P CO2=40 mmHg, hemoglobin fully saturated with O2, T = 37 C). Essentially, the use of standard conditions has the effect of reversing the compensatory effect of respiration and thus to make more evident the eventual presence of a metabolic component in the pH unbalance. In order to gain an understanding of the concept of standard pH and those that derived from it (standard bicarbonate and base excess) one should consider that CO2 behaves as an acid because of the reaction CO2 + 2 H2O <==> HCO3- + H3O+. Thus, if P CO2 < 40 mmHg, CO2 is absorbed during equilibration, and standard pH < pH. On the contrary, if P CO2 > 40 mmHg, CO2 is released during equilibration, and standard pH > pH. With the use of three parameters (total CO2, pH, and standard pH), one obtains a better description of the underlying clinical condition, according to the following table:
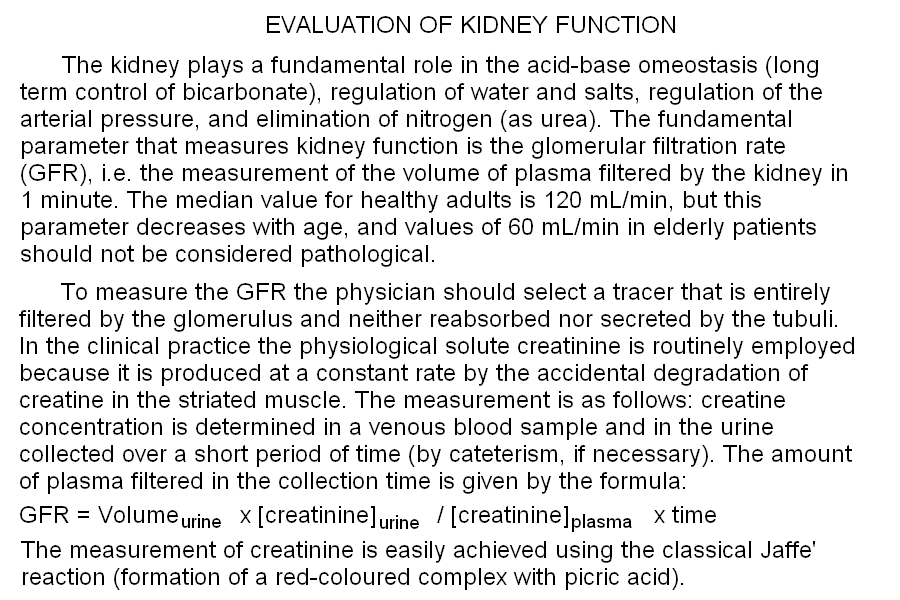
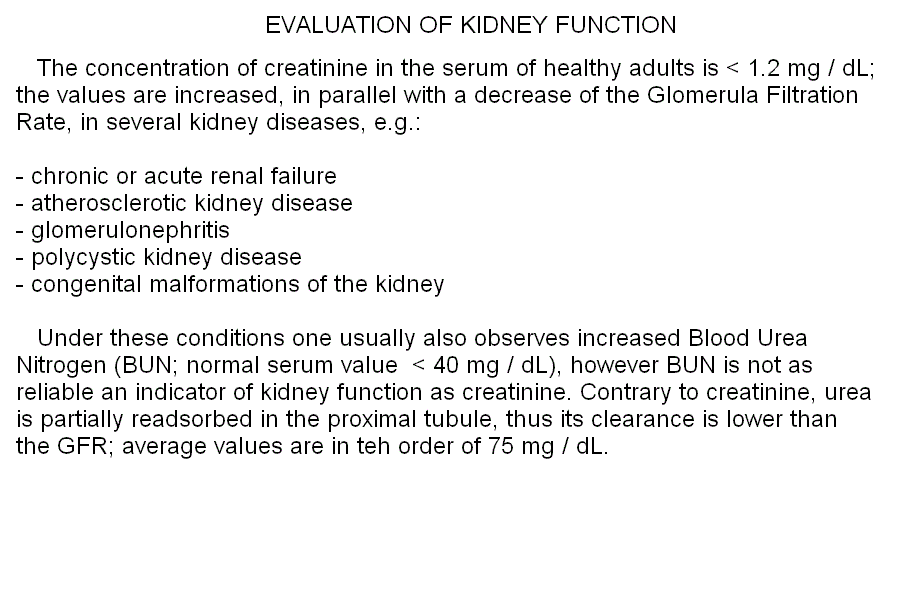
Standard bicarbonate, a concept developed by Astrup and Siggaard Andersen in 1957, is the concentration of bicarbonate one measures when a sample of arterial blood is equilibrated under standard conditions. The rationale of this procedure is that of setting the concentration of one of the components of the major blood buffer (CO2) and determinining that of the other. The number one measures is not the same one would obtain in a standard hemogas analysis, given that part of the bicarbonate originally present in the blood may be lost as CO2 (if the P CO2 was higher than 40 mmHg) or part of the gas may be absorbed and converted to bicarbonate. The standard bicarbonate measures the metabolic component of the acid-base balance of the blood and corrects for respiratory compensation. It can substitute for standard pH. Base excess, again by Astrup and Siggaard Andersen, is the amount of strong acid (or base, in which case the resulting value is negative) required to restore the normal pH of 1 L of blood sample equilibrated under standard conditions. Standard base excess is the same concept except that erythrocytes are partially removed, to a final Hb content of 5 g/dL. BE (or SBE) is another indicator of the metabolic component of the disturbance: acidosis in the presence of positive base excess is an indication of respiratory acidosis. Notice that if acidosis is present (i.e. pH<7.4) and the base excess is positive (i.e. acid is required to restore the pH to 7.4), this implies that the process of equilibrating blood under standard conditions causes the pH to raise above 7.4 (in respiratory acidosis P CO2 > 40 mmHg; thus equilibration with standard P CO2 removes CO2 and bicarbonate). By contrast, acidosis in the presence of a negative base excess (base deficit) indicates an important metabolic component, with respiratory compensation. Base excess provides information analogous to, but more quantitative than, standard pH and standard bicarbonate (see the above Table "Typical laboratory data for acidoses and alkaloses"). Advanced, 5-parameters interpretation of the arterial hemogas analysis. 1) The usual 4-parameters evaluation is carried out as above. 2) The standard pH, or standard bicarbonate, or base excess is added to the picture and interpreted as in the Table above. The kidney plays a fundamental role in the acid-base omeostasis (long term control of bicarbonate), regulation of water and salts, regulation of the arterial pressure, and elimination of nitrogen (as urea). The fundamental parameter that measures kidney function is the glomerular filtration rate (GFR), i.e. the measurement of the volume of plasma filtered by the kidney in 1 minute. The median value for healthy adults is 120 mL/min, but this parameter decreases with age, and values of 60 mL/min in elderly patients should not be considered pathological. To measure the GFR the physician should select a tracer that is entirely filtered by the glomerulus and neither reabsorbed nor secreted by the tubuli. For experimental studies the polysaccaride inulin is used, but in the clinical practice the physiological solute creatinine is routinely employed because it is physiologically produced at a constant rate by the accidental degradation of creatine in the striated muscle. The measurement is as follows: creatine concentration is determined in a venous blood sample and in the urine collected over a short period of time (by cateterism, if necessary). The amount of plasma filtered in the collection time is given by the formula:  Home of this course Slides for this lecture:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||