| Course of LABORATORY MEDICINE Plasma Proteins The human blood plasma contains proteins at the total concentration of approx. 7 g/dL. Alterations in the concentration of plasma proteins and their relative abundance may vary in the course of diseases and provides important diagnostic clues. Classification of the proteins present in the plasma is quite complex due to their large variety. It is important to distinguish between those proteins that are present in the plasma because this is where they exert their physiological function, and those that are accidentally present in the plasma because of the death of the cells in whose cytoplasm they exert their physiological function. Since all cells of our body are subject to some turnover, the finding of low levels of intracellular enzymes in the plasma is of no diagnostic significance; but large increases of these levels suggest acute disease with cell death (e.g. myocardial infarction, viral hepatitis, etc.). Most of the proteins that are physiologically present in the blood plasma are produced by the liver, the most relevant exception being that of the antibodies that are produced by plasma cells.
Degradation and removal of the plasma proteins may occur via at least three different mechanisms: (i) most of the proteins physiologically present in the plasma, and some of those present because of cell death are removed by macrophages, Kuppfer cells and other scavenging cells, digested to aminoacids and recycled in the blood in this form; (ii) low molecular weight proteins (MW < 30-50 KDa) are filtered by the kidney and appear in the urine; many of the proteic hormones and of the proteins released by cell death fall in this category; (iii) a small number of plasma proteins are removed beacuse of specific consumption (e.g. fibrinogen) or transferred to the extracellular fluids. METHODS FOR THE STUDY OF PLASMA PROTEINS Total protein concentration is estimated using the reactions characteristic of some aminoacids or of the peptide bond, and applying specifci conversion factors. The classic method of Kjeldahl, consisting in the liberation of nitrogen as ammonia and titration of the latter compound is not used any more because it is expensive and time consuming. Biuret is a copper reagent that allows complexation of the metal with the peptide bond. The resulting product is blue colored and its concentration can be determined spectrophotometrically. The method of Lowry measures the Tyrosine concentration using a reagent for phenols (phosphotungstomolibdic acid), that again yields a blue complex whse concentration is measured by absorbance spectrophotometry. The average value of total protein concentration in healthy subjects is 7 g/dL. Electrophoresis is the method of choice for protein fractionation: 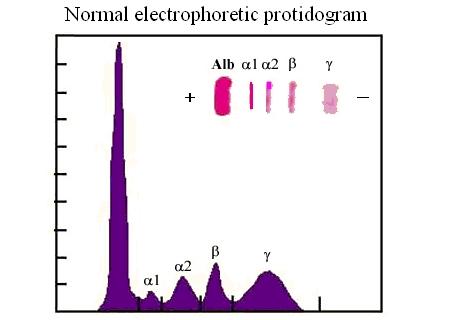 A tiny amount of the serum or plasma under analysis is adsorbed onto a cellulose acetate strip equilibrated in an alkaline buffer; the positive and negative electrodes are connected to the ends of the strip and current is applied in order to attract the proteins towards the positive electrode. The more negatively charged is the protein, and the smaller its dimension, the faster it moves (it should be remembered that at alkaline pH ionizable groups of the aminoacid side chains are deprotonated, hence the negative charge of the protein). The proteins adsorbed on the strip are fixed and stained with a suitable dye and their concentration is measured using a reflectance spectrometer. The resulting graph can be used to quantitate the reflectance peaks, hence the relative protein concentrations in the serum or plasma. Albumin is the fastest moving protein and forms a sharp band; since this band consists essentially of a single chemical species its quantitation is very straightforward. The alpha1 and alpha2 globulin fractions are less mobile than albumin, followed by the beta globulin fraction. These fractions do not correspond to pure chemical species, and many different proteins co-migrate in the alpha1, alpha2 and beta band. Because of this reason quantitation of these bands in the electropherogram only gives a very generic information. The slowest protein component is costituted by the gamma globulins and essentially corresponds to the antibodies; it is increased in the course of chronic bacterial infections (polyclonal increase) or in the course of multiple myeloma (forming a monoclonal sharp peak).
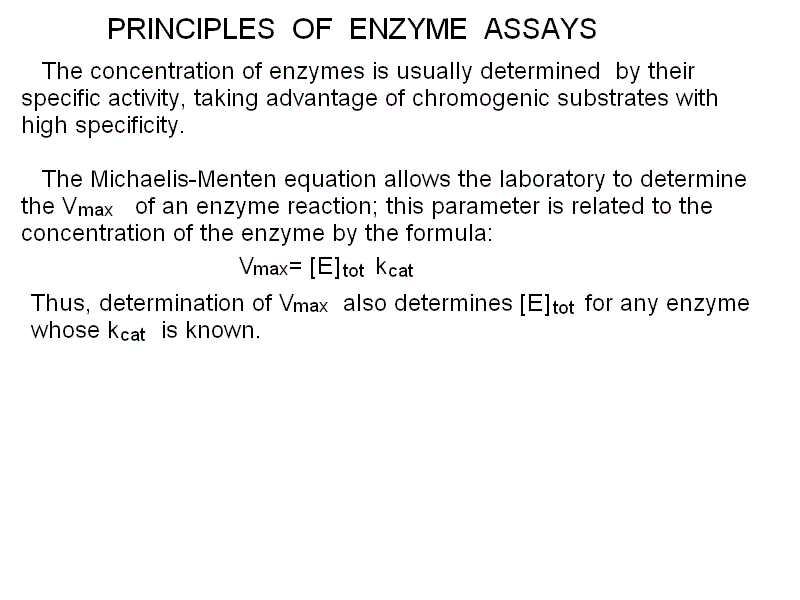
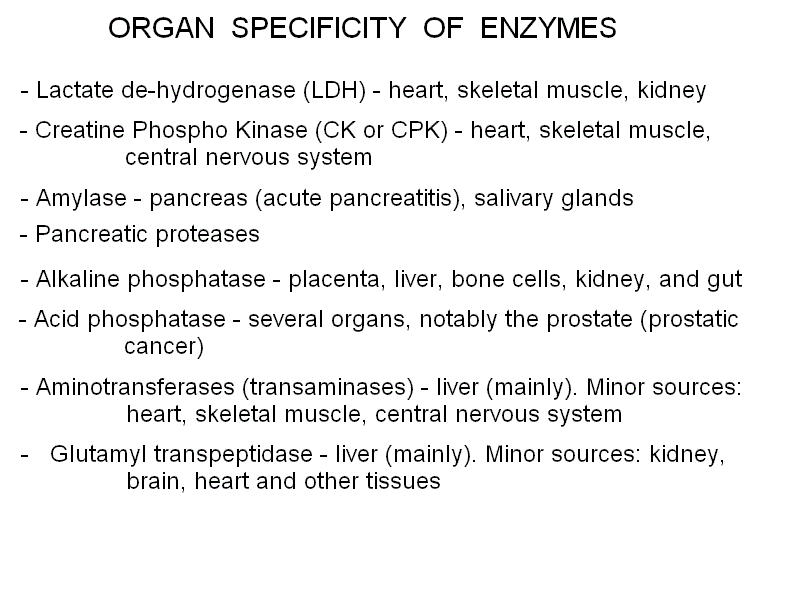
A LIST OF SOME IMPORTANT PROTEINS IN THE PLASMA Albumin is the protein present at the highest concentration in the blood plasma (3.5-5.5 g/dL, accounting for over than half the total protein concentration). Albumin is a non-specific carrier of poorly soluble substances (e.g. unconjugated bilirubin, steroid hormones, heme, drugs, etc.) and is produced by the liver. Several genetic variants are known. Albumin is soluble in distilled water as well as in physiological saline solutions, and is rich in acidic aminoacids that confer to it a low isoelectric point (approx. 4.5); as a consequence in the electrophoresis is migrates fast towards the positive electrode and very few plasma proteins move faster than albumin (e.g. pre-albumin, the carrier specific for thyroid hormones). Albumin is produced by the liver and its circulating half-life is approx. 20 days; cells that require aminoacids for protein synthesis may uptake and digest albumin. The concentration of albumin is increased in all clinical conditions causing hemoconcentration (e.g. vomiting, diarrhoea, severe burns, etc.); it is decreased during chronic liver diseases (because of impaired biosynthesis), in nephrotic syndrome (because of loss in the urine) and in severe malnutrition. Since albumin is the major determinant of the colloido-osmotic pressure of the blood hypoalbuminemia is usually associated with oedema and ascites, especially if hypoalbuminemia is due to a liver disease causing portal hypertension (e.g. cirrosis). Measurement of albumin concentration is directly made on the electrophoretic protidogram, given the characteristic electrophoretic mobility and the great relative abundance. Haptoglobin is produced by the liver. It binds to free hemoglobin produced by hemolysis of red blood cells preventing the reaction of the heme iron with xygen, that may produce reactive oxygen species (ROS). Hemopexin is produced by the liver. It binds to the free heme and prevents its reactions with oxygen. Transferrin is produced mainly by the liver and has the function of transporting iron. Caeruloplasmin is produced bythe liver and has the physiological function of transporting copper. In the electrophoresis it migrates with the alpha2 globulin fraction Protease inhibitors. The blood plasma contains several proteins that act as inhibitors of serine proteases, in particular of trypsin, chymotrypsin and related pancreatic enzymes. These are collectively called serpins (for SERine Protease INhibitors). Pancreatic enzymes may be occasionally readsorbed in the blood. Since these enzymes are able to promote the coagulation of fibrinogen and would cause diffuse intravascular coagulation and several other adverse effects, it is important that the plasma is able to neutralize their activity. The most important protease inhibitors physiologically present in the blood are alpha1 antitrypsin, alpha1 antichymotrypsin, and alpha2 macroglobulin. Complement is a system of several plasma proteins that migrate in the electrophoretic fraction of the beta-globulins and plays a function in the defence from infections. The proteins of complement circulate in the blood in the form of inacteive precursors, and when activated they acquire enzymatic activities (mainly as esterases) that allow them to damage the lipid bilayer of the (bacterial) cell membrane. Excessive response of the complement may also damage the cells of the organism. Complement is activated by the antigen-antibody complexes; by the C-reactive protein; and by some fungi, bacteria and viruses. There is also an alternative activation pathway, taht does not involve immunocomplexes and requires insteda the specific serum protein Properdin. Several genetic defects of complement are known that cause increased propensity to infectious diseases. Acute Phase Proteins are produced and released in the blood serum during a variety of pathological stimuli causing cell necrosis in any tissue. Common causes may be inflammation, infection, and cancer. During acute phase reactions some plasma proteins may appear decreased (e.g. albumin), partly due to the relative increase of acute phase proteins. Other increase slightly (e.g. complement components C3 and C4; protease inhibitors; haptoglobin; caeruloplasmin). AThe concentration of a few proteins increases very markedly (up to 1000-fold): C-reactive protein and serum amyloid A (SAA). Acute Phase Proteins (especially C-reactive protein and SAA) are generic indicators of inflammation and tissue damage (like inbcreased ESV) and indicate further diagnostic screenings; however they give little information on the possible underlying pathology. In children and young adults common conditions to consider are rheumatic fever, rheumatoid arthritis and other autoimmune diseases, bacterial infections; in older subjects cancer should be also considered. C-reactive Protein is produced by the liver and is a pentamer of 23 kDa subunits. It derive its name from its ability to bind to the C-polysaccharide of the Streptococci. In healthy adults its serum concentration is <1 mg/dL (or <90 nM); during acute inflammation its concentration increases very significantly (up to 100- to 1000-fold). Its function is to combine with several infectios agents and to activate complement. Serum Amyloid A is a HDL apolipoprotein, present in the serum of healthy adults at a concentration <3 mg/dL. Its physiological function is the transport of liposoluble substances (notably oxidized cholesterol derivatives) to the liver. Immunoglobulins are produced by the plasma cells and migrate in the fraction of the gamma-globulins (actually this fraction only contains immunoglobulins). As already stated, in addition to the proteins that exert a physiological function there, the human blood plasma (or serum) may contain enzymes released by the death of cells of every organ of the organism. In the healthy individual the plasma concentration of these enzymes is low, but non-zero, because of the physiological turnover of the cells of every tissue. In several acute and chronic diseases (including inflammation, infarction and cancer), the concentration of some of these enzymes may markedly increase, thus providing important diagnostic clues. Enzymes are usually quite easy to quantitate, thanks to their specific activity: a chromogenic substrate (i.e. a physiological or artificial substrate whose transformation by the enzyme causes a change in its spectrocopic properties) is added to the patient's plasma, and the velocity of its conversion to the product is followed by absorbance spectroscopy. The rate of substrate transformation is correlated to the enzyme concentration by the Michaelis and Menten equation: v = [E] kcat [S] / (KM + [S]) where [S] is the substrate concentration and kcat and KM are the kinetic parameters characteristic of the enzyme. Given that the precise values of kcat and KM may vary because of the experimental conditions, the laboratory standardizes its own process using purified commercial samples of each enzyme. When measured with functional methods, the enzyme concentration is expressed in Standardized Units per mL (U/mL). The effective value of the Standardized Unit varies with different enzymes (since each requires its own experimental conditions), but the concept is always the same: a Unit is the amount of enzyme that transforms one mMole (or microMole or other amount) of substrate in one minute (or one second). Other methods to quantitate the enzymes' concentration in the blood plasma are by electrophoresis, and by immuno-assays (these may be expensive since they require that the laboratory buys specific antibodies for each enzyme of clinical interest). In some instances the enzyme of interest may have special spectroscopic properties (e.g. cytochrome c contains a heme group), allowing direct measurement by means of absorbance or fluorescence spectroscopy. Isoenzymes. Some enzymes are coded by more than a single gene and exist in the for of several variants called isoenzymes. These isoforms of the same enzyme have specific tissue distributions and patterns. A typical example is observed in the case of Lactate dehydrogenase that is a tetramer made up by two types of subunits, called H (for heart, prevalent in the heart cells) and M (for muscle, prevalent in muscle cells). The H and M subunits can assemble freely so that the tetrameric enzyme may have formulas such as H4, H3M1, ..., M4. From the physiological point of view these variants behave very similarly, but from the clinical standpoint their identification is important for diagnosis. Some relevant enzymes, together with their diagnostic relevance, are described below. Lactate dehydrogenase (LDH) is a tetramer made up of two types of subunits, called H and M. The enzyme catalyzes the reversible reduction of piruvate to lactate that occurs in anaerobic glycolysis (i.e. when glycolysis exceeds the ability of the mitochondrion to oxidize the acetyl-CoA derived from the decarboxylation of piruvate, due to insufficient availability of oxygen; the most typical condition is strenuous muscular effort): CH3-CO-COOH + NADH + H+ <==> CH3-CHOH-COOH + NAD+ Lactate dehydrogenase is present in many metabolically active tissues, e.g. the heart, skeletal muscle, kidney, and is increased in clinical conditions causing the death of these tissues (e.g. myocardial infarction, renal infarction, crush syndrome with multiple muscular lesions). The characterization of isoenzyme variants is clinically relevant: the two types of polypeptide chains may form pure or mixed tetramers with formulas H4 (called LDH1), H3M (LDH2), H2M2 (LDH3), HM3 (LDH4), M4 (LDH5). In cardiac and renal lesions tetramers containing prevalently H-type subunits predominate, whereas the opposite holds in the case of lesions of the liver or the skeletal muscle. Creatine (phospho)kinase (CK or CPK) is a dimeric enzyme; two isoforms exist called M (for muscle) and B (for brain); it characteristic or the myocardium (CPK2 corresponding to the dimer BM); of the skeletal muscle (CPK3, MM); and of the Central Nervous System (CPK1, BB). CK catalyzes the reversible exchange of phosphate groups between ATP and creatine; phosphocreatine acts as a reservoir of high energy phosphate groups, especially useful to sustain muscular contraction during anaerobic strenuous muscular effort. The CK concentration in the serum of healthy adults is < 30 U/mL and is significantly increased in myocardial infarction and in acute disorders of the muscles or the Central Nervous System(e.g. traumas, cerebral infarction, etc.). Amylase is produced by the salivary glands and by the pancreas; its function is the hydrolysis of starch and glycogen of alimentary origin. Its serum concentration may be strongly increased in the course of acute pancreatitis, but this finding is inconstant since during this disease the pancreatic proteases (most notably trypsin and chymotrypsin) are also released and may degrade the other pancreatic enzymes before they reach the blood. The same applies to lipase (which is not produced by the salivary glands). Two isoenzymes of amylase exhist, called S (present primarily in the salivary glands and the lung) and P (in the pancreas). Pancreatic proteases: Trypsin, Chymotrypsin, Elastase and other enzymes are secreted in the gut and play the physiological function of digesting alimentary proteins; they are released in the blood during episodes of acute or chronic pancreatitis. They cause major symptoms (mortality of acute pancreatitis may exceed 30%) since they are able to digest many proteins present in the plasma: e.g. thay may cause Disseminated Intravascular Coagulation becuase of direct conversion of fibrinogen into fibrin and because of the activation of thrombin; transformation of angiotensinogen to angiotensin and degradatio of the latter; etc. Free Pancreatic proteases do not usually attain very high levels in the plasma in their active form since they are counteracted by Serpins (see above). Alkaline phosphatase is produced by several organs, including the placenta, liver, bone cells, kidney, and gut. The enzyme's activity is poorly specific as it hydrolyzes the phosphoric esters of several alcohols (including phosphorylated proteins). The enzyme is released in the blood by all diseases causing death of the cells of these organs. Cancer of these organs is an important cause of increased serum alkaline phosphatase, due to the fact that cancer cells are subject to rapid turnover and many of them die because of poor oxygenation of the cancer tissue. Acid phosphatase is produced by several tissues, notably by the prostate and is significantly increased in advanced cases of prostatic cancer, especially if metastases are present. Other conditions causing increased concentration of acid phosphatase are prostatic hyperplasia, multiple myeloma, Gaucher's disease, and hemolytic anemia. The physiological function and substrates of this enzyme are very similar to those of alkaline phosphatase, from which it differs because it is most active at acidic pH Aminotransferases (transaminases) are characteristic of liver cells. They catalyze the reversible exchange of amino-groups between alpha-chetoacids and alpha-aminoacids: e.g. the reaction catalyzed by ALT is the reversible conversion of alpha-chetoglutaric acid and alanine into glutamic acid and piruvic acid, as follows: COOH-CH2-CH2-CO-COOH + CH3-CHNH2-COOH <==> COOH-CH2-CH2-CHNH2-COOH + CH3-CO-COOH The most important aminotransferases are Alanine Aminotransferase (ALT; also called Glutamate-Pyruvate Transaminase, GPT or sGPT) and Aspartate Aminotransferase (AST; also called Glutamate-Oxaloacetate Transaminase, GOT or sGOT). Other tissues containing these enzymes are the heart, skeletal muscle and the Central Nervous System The reference values of these enzymes are <40 U/mL. The transaminases are elevated in several acute conditions such as myocardial or cerebral infarctions; however they attain their highest levels and are diagnostic for liver diseases. In acute viral hepatitis their serum concentration may usually 500 U/mL; in chronic conditions (e.g. alcoholic liver disease, cirrosis) they rarely exceed 300 U/mL. Under most circumstances, increased serum concentration of ALT and AST is associated with other signs of liver disease (e.g. jaundice-hyperbilirubinemia; itch due to reabsorption of bile salts; pale stools and dark urine). gamma-glutamyl transpeptidase (gammaGT) Gamma-glutamyl transpeptidase (or gamma-glutamyl transferase, gammaGT) is an enzyme that transfers a residue of glutamic acid from a donor (usually glutathione) to an acceptor (a free aminoacid or a xenobiotic). It is involved in several detoxification reactions in the liver, kidney, brain, heart and other tissues. From a clinical standpoint, elevated serum gammaGT is usually an indication of liver disease. Principles of clinical investigation: the typical screening of serum enzymes involves measuring the activity of six enzymes, that taken together provide an important clinical picture, and indicate the direction of further investigation: ALT, AST, LDH, CPK, alkaline phosphatase (ALP), possibly acidic phosphatase (ACP), and γ-glutamyl transpeptidase (γ-GT). The pattern of the increase of concentration of (some of) these enzymes provides information on possible diseases of the liver, heart, hematopoietic tissue, bone, skeletal muscle and prostate and may raise suspicion of acute inflammatory or ischemic diseases (e.g. viral hepatitis, myocardial infarction) or cancer.
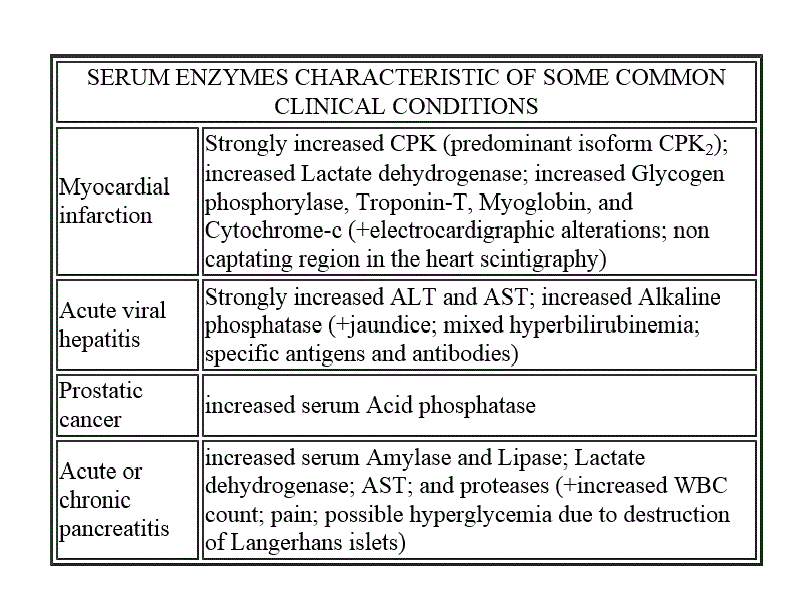
*: lipases can be released by the endothelial cells under heparin treatment. When measuring serum enzymes one is effectively searching for indication of cell death in a specific tissue or organ; thus enzymatic measurements should be coupled to measurements of organ function: - Kidney: kidney damage (aterosclerotic; neoplastic; due to infection or to autoimmune reactions) is revealed by increased levels of ALP and γ-GT; it should be complemented with the measurement of glomerular filtration rate (GFR) usually estimated by the clearance of creatinine (normal values 80-12 mL/min.), and by the measurement of serum concentrations of urea (normal value < 50 mg/dL) and creatinine (normal value < 1.5 mg/dL). - Liver: liver damage (toxic; neoplastic; due to viral infection; etc.) is revealed by increased levels of AST, ALT, ALP, and γ-GT. It is usually associated to hyperbilirubinemia and jaundice (normal value of bilirubin < 1 mg/dL). In advanced cases of chronic liver failure hypoalbuminemia (normal value > 3.5 g/dL) and hyperammonemia (normal value 10-35 μmol/L). - Myocardial infarction causes a complex enzymatic pattern (see Table), associated to ECG alterations. In severe cases arrhythmias may be present, possibly complicated by venous hypertension (pulmonary or systemic). Ultrasonography reveals an area of the myocardium which does not contract and scintigraphy reveals that the same area does not uptake the tracer. Coronarography may reveal the obstruction. The enzymatic pattern of the heart may be quite similar to those of the brain, the skeletal muscle, and to some extent the smooth muscle. - Cancer has high cellular turnover and may mimic the enzymatic pattern of the tissue from which it originates. Special attention should be paid to prostate cancer (increased ACP and PSA may be early signs), and to hematological cancers (leukemias and lymphomas may cause increased ALP and lysozyme; diagnosis is established by the finding of cancer cells circulating and/or in the bone marrow).
Macroenzymes: enzymes in the blood plasma may form high molecular weight complexes with other macromolecules; these are called macroenzymes. As a general rule the clinical significance of the macroenzyme is the same as that of the enzyme; however the half life in the circulation may be increased, thus macroenzymes may lead to an overestimation of the severity of the patient's condition; in some cases the persistence is so increased as to cause an abnormal result in a healthy individual. Examples of macroenzymes. Commonly observed macroenzymes are the complexes of enzymes with immunoglobulins (usually IgG; amylase and LDH preferentially bind to IgA). Binding occurs at the antigen binding site, thus the presence of macroenzymes may be assimilated to a form of immune self reactivity, possibly caused by cross reactivity with the homologous enzymes from occasionally encountered pathogens, or by the fact that the intracellular enzymes are sequestered antigens for which the immune system has no tolerance. The presence of macroenzymes of this type is a benign condition, generally devoid of special clinical implications, except for the possible diagnostic and therapeutical errors. Another example of macroenzyme is the complex of serine proteases with alpha2 macroglobulin (a specific protease inhibitor); the presence of this macroenzyme may indicatea mild episode of pancreatitis and justifies further investigation. Creatine kinase may form homopolymers, that qualify as macroenzymes; these may suggest the presence of severe liver disease or colon adenocarcinoma, thus an accurate clinical investigation of the patient is mandatory. Home of this course Slides of this lecture:
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||